
Concept explainers
Interpretation:
The relation between the arrangements of electrons in an atom with the location of the atom on the periodic table is to be described.
Concept introduction:
A periodic table can be divided into four zones, s block, p block, d block and f block. The element belonging to a particular block will have its outermost electron in that particular subshell. For example, in the case of s block elements, the outermost electrons will occupy the subshell. Elements in p block will have its outermost electrons in p subshell.
Explanation of Solution
Electrons revolve around the nucleus in orbits. These orbits are called shells. The shells are numbered as n = 1, 2, 3, 4, and so on. In an
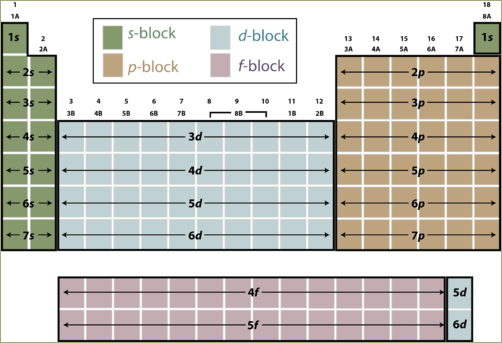
Any element located in the s block will have its outermost electron(s) in s subshell. On moving across the periodic table from one element to the next, one additional proton and one additional electron are added, along with one or more neutrons. Each additional electron goes into a specific subshell. If an atom is located in the p block of the table, the last electron is placed into a p subshell. If an atom is located in the d block of the table, the last electron is placed into a d subshell and so on.
The element belonging to a particular block will have its outermost electron in that particular subshell.
The elements in each block have related properties. The elements in the s-block are reactive metals. The elements in the d-block tend to form colorful compounds that are used as pigments. The elements in the p-block form colorless compounds.
By knowing the position of an element on a periodic table, one can predict the arrangement of electrons. The periodic table provides the information needed to write electronic configurations.
Chapter U1 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Essential Biology with Physiology (5th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Anatomy & Physiology (6th Edition)
Microbiology with Diseases by Body System (5th Edition)
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
- What is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forward
- Predict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forward
- Predict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forwardYou are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





