
Interpretation:
The revised plum pudding model that includes protons needs to be explained.
Concept introduction:
According to the Thomson experiment, if the electric discharge from a high potential source is passed through a discharge tube evacuated to pressure around 0.1mm or less, rays emitted from the cathode. These are called cathode rays. These rays travel in straight lines at right angles to the cathode surface. They produce mechanical motion in small paddle wheel placed in their path indicating that that are material particles. These particles are deflected form their path by electric path and magnetic fields showing that they are electrically charged. The direction of deflection indicates that they are negatively charged . These particles are called electrons.
Also, in the experiment, if the discharge tube contains perforate cathode which emitted another type of radiation which start from the side by side of the anode and pass through the holes of the cathode.
The radiation carrying the positive charge is positive rays or anode rays. Further it is discovered that there are positively charged particles in these rays. These positive charged particles are named protons.
Explanation of Solution
The plum pudding model of an atom explains that electrons are surrounded by a positively charged volume. In the model, plums are refereed to negatively charged particles and pudding is positively charged. This model is known as Thomson’s plum pudding model.
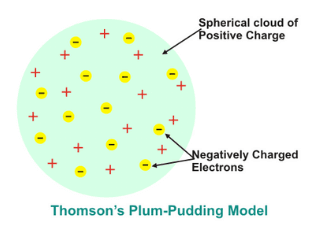
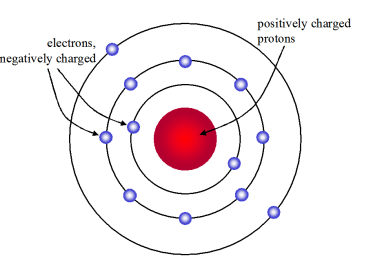
Now, if protons where discovered after electrons, the plum pudding model can be revised with positive charged particles at the center (occupying very less space) which are surrounded by negatively charged particles.
The number of positive and negative charged particles will be same as atom is neutral in nature. The revised plum pudding model will be:
Chapter U1 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Microbiology: An Introduction
Brock Biology of Microorganisms (15th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Cosmic Perspective Fundamentals
Campbell Biology: Concepts & Connections (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Iarrow_forwardDraw the anti-Markovnikov product of the hydration of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for esc esc ☐ Explanation Check F1 1 2 F2 # 3 F3 + $ 14 × 1. BH THE BH3 2. H O NaOH '2 2' Click and drag to start drawing a structure. F4 Q W E R A S D % 905 LL F5 F6 F7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility < & 6 7 27 8 T Y U G H I F8 F9 F10 F11 F12 9 0 J K L P + // command option Z X C V B N M H H rol option commandarrow_forwardAG/F-2° V 3. Before proceeding with this problem you may want to glance at p. 466 of your textbook where various oxo-phosphorus derivatives and their oxidation states are summarized. Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14: -0.93 +0.38 -0.50 -0.51 -0.06 H3PO4 →H4P206 →H3PO3 →→H3PO₂ → P → PH3 Acidic solution Basic solution -0.28 -0.50 3--1.12 -1.57 -2.05 -0.89 PO HPO H₂PO₂ →P → PH3 -1.73 a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the formation and reduction of H4P206 (-0.93/+0.38V). Calculate the values of AG's for both processes; comment. (3 points) 0.5 PH P 0.0 -0.5 -1.0- -1.5- -2.0 H.PO, -2.3+ -3 -2 -1 1 2 3 2 H,PO, b) Frost diagram for phosphorus under acidic conditions is shown. Identify possible disproportionation and comproportionation processes; write out chemical equations describing them. (2 points) H,PO 4 S Oxidation stale, Narrow_forward
- 4. For the following complexes, draw the structures and give a d-electron count of the metal: a) Tris(acetylacetonato)iron(III) b) Hexabromoplatinate(2-) c) Potassium diamminetetrabromocobaltate(III) (6 points)arrow_forward2. Calculate the overall formation constant for [Fe(CN)6]³, given that the overall formation constant for [Fe(CN)6] 4 is ~1032, and that: Fe3+ (aq) + e = Fe²+ (aq) E° = +0.77 V [Fe(CN)6]³ (aq) + e¯ = [Fe(CN)6] (aq) E° = +0.36 V (4 points)arrow_forward5. Consider the compounds shown below as ligands in coordination chemistry and identify their denticity; comment on their ability to form chelate complexes. (6 points) N N A B N N N IN N Carrow_forward
- 1. Use standard reduction potentials to rationalize quantitatively why: (6 points) (a) Al liberates H2 from dilute HCl, but Ag does not; (b) Cl2 liberates Br2 from aqueous KBr solution, but does not liberate C12 from aqueous KCl solution; c) a method of growing Ag crystals is to immerse a zinc foil in an aqueous solution of AgNO3.arrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 3 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? N N H3O+ +R + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. fmarrow_forward
- The product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH3CN H+ N Click and drag to start drawing a structure. 5arrow_forwardAssign this HSQC Spectrum ( please editing clearly on the image)arrow_forward(a 4 shows scanning electron microscope (SEM) images of extruded actions of packing bed for two capillary columns of different diameters, al 750 (bottom image) and b) 30-μm-i.d. Both columns are packed with the same stationary phase, spherical particles with 1-um diameter. A) When the columns were prepared, the figure shows that the column with the larger diameter has more packing irregularities. Explain this observation. B) Predict what affect this should have on band broadening and discuss your prediction using the van Deemter terms. C) Does this figure support your explanations in application question 33? Explain why or why not and make any changes in your answers in light of this figure. Figure 4 SEM images of sections of packed columns for a) 750 and b) 30-um-i.d. capillary columns.³arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





