
Concept explainers
(a)
Interpretation:
The two different versions of periodic table need to be represented by explaining similarity between them.
Concept introduction: There are two version of the periodic table −one of them proposed by Russian Chemist Dmitri Mendeleev’s known as the old Periodic table. Later on due to some demerits new form of Periodic table Modern form of the periodic table. A total of 118 elements is arranged in the form of a table. The periodic table has horizontal and vertical rows-Groups and Periods.
Answer to Problem 7E
Elements are arranged on the basis of
Explanation of Solution
In both forms of periodic table, elements are arranged in groups and periods.
Periodic table in which elements are arranged in increasing order to atomic mass is as follows:
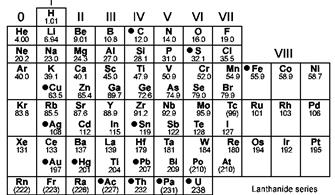
The periodic table where elements are arranged in increasing order of atomic number is as follows:

(b)
Interpretation:
The two different versions of periodic table need to be represented by explaining difference between them.
Concept introduction: There are two version of the periodic table −one of them proposed by Russian Chemist Dmitri Mendeleev’s known as the old Periodic table. Later on due to some demerits new form of Periodic table Modern form of the periodic table. A total of 118 elements is arranged in the form of a table. The periodic table has horizontal and vertical rows-Groups and Periods.
Explanation of Solution
Elements are arranged on the basis of atomic mass and on the basis of the atomic number in two different periodic tables.
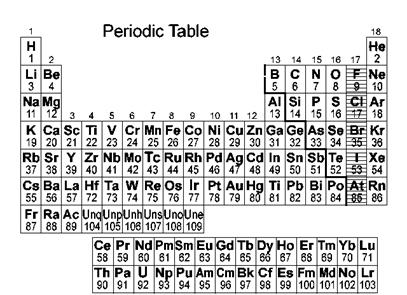
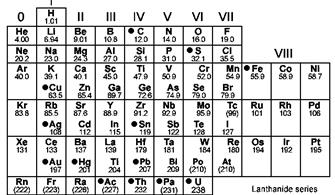
The noble gases or neon gases are present in group zero in the periodic table where elements are arranged in increasing order of atomic masses and they are present in group 18 when elements are arranged in increasing order of atomic number.
Chapter U1 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Chemistry: The Central Science (14th Edition)
College Physics: A Strategic Approach (3rd Edition)
Applications and Investigations in Earth Science (9th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology with Diseases by Body System (5th Edition)
- Using iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forwardDraw the product formed when the following pair of compounds is treated with NaOEt in ethanol. + i CNarrow_forwardI need help with the followingarrow_forward
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





