
(a)
Interpretation:
The number of electrons in an atom of Carbon (C) and Silicon (Si) element needs to be determined.
Concept introduction:
An atom is considered as the basic unit of matter. It is composed of three sub-atomic particles; electrons, protons and neutrons.
Electrons are placed in certain energy levels which are called as shell. The outermost shell of an atom is called as valence shell and the electrons are called as valence shell electrons. These valence shell electrons take part in all
(a)
Answer to Problem 6E
Carbon = 6 electrons
Silicon = 14 electrons
Explanation of Solution
Both carbon and silicon are member of same group of periodic table that is 14th group. The
In a neutral atom, the number of protons (atomic number) is always equal to the number of electrons because neutrons are neutral particles and the charge balance must be from the charge of protons and electrons.
(b)
Interpretation:
The shell model for an atom of Carbon (C) and Silicon (Si) element needs to be determined.
Concept introduction:
An atom is considered as the basic unit of matter. It is composed of three sub-atomic particles; electrons, protons and neutrons.
Electrons are placed in certain energy levels which are called as shell. The outermost shell of an atom is called as valence shell and the electrons are called as valence shell electrons. These valence shell electrons take part in all chemical reactions and are also involved in chemical bonding.
(b)
Answer to Problem 6E
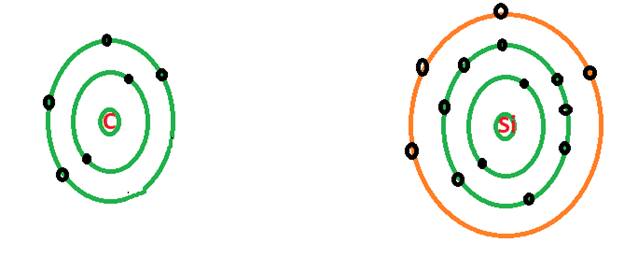
Explanation of Solution
Both carbon and silicon are member of same group of periodic table that is 14th group. The atomic number of carbon is 6 and the atomic number of silicon is 14.
The shell model of an atom represents the number of electrons in each shell of the atom. The shell model of an atom indicates the electronic distribution of electrons in an atom.
(c)
Interpretation:
The number of valence electrons in an atom of Carbon (C) and Silicon (Si) element needs to be determined.
Concept introduction:
An atom is considered as the basic unit of matter. It is composed of three sub-atomic particles; electrons, protons and neutrons.
Electrons are placed in certain energy levels which are called as shell. The outermost shell of an atom is called as valence shell and the electrons are called as valence shell electrons. These valence shell electrons take part in all chemical reactions and are also involved in chemical bonding.
(c)
Answer to Problem 6E
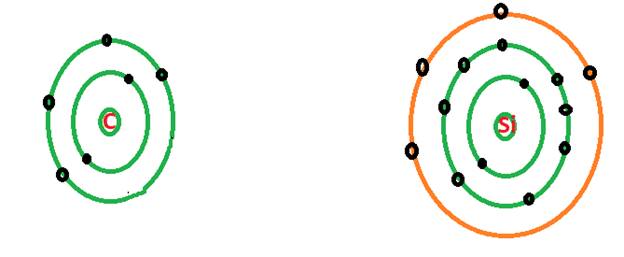
C = 4 valence electrons
Si = 4 valence electrons
Explanation of Solution
Both carbon and silicon are member of same group of periodic table that is 14th group. The atomic number of carbon is 6 and the atomic number of silicon is 14.
The valence shell electrons are the electrons in the outer most shell of an atom. Both C and Si belong to same group in the periodic table and the elements in the same group have same valence shell electronic configuration. There are 4 electrons in the valence shell of both C and Si atom as shown in the shell model.
(d)
Interpretation:
The number of core electrons in an atom of Carbon (C) and Silicon (Si) element needs to be determined.
Concept introduction:
An atom is considered as the basic unit of matter. It is composed of three sub-atomic particles; electrons, protons and neutrons.
Electrons are placed in certain energy levels which are called as shell. The outermost shell of an atom is called as valence shell and the electrons are called as valence shell electrons. These valence shell electrons take part in all chemical reactions and are also involved in chemical bonding.
(d)
Answer to Problem 6E
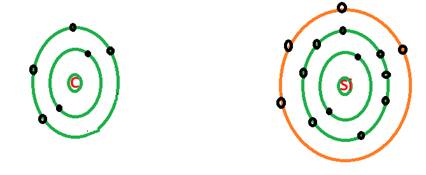
C = 2 valence electrons
Si = 10 valence electrons
Explanation of Solution
Both carbon and silicon are member of same group of periodic table that is 14th group. The atomic number of carbon is 6 and the atomic number of silicon is 14. There are 4 electrons in the valence shell of both C and Si atom as shown in the shell model. Thus, the remaining electrons must be in innermost shell which are called as core electrons.
The number of core electrons in:
C = Atomic number − valence electrons = 6-4 = 2 electrons
Si = Atomic number − valence electrons = 14-4 = 10 electrons
(e)
Interpretation:
The reason of similar properties of carbon and silicon needs to be determined.
Concept introduction:
An atom is considered as the basic unit of matter. It is composed of three sub-atomic particles; electrons, protons and neutrons.
Electrons are placed in certain energy levels which are called as shell. The outermost shell of an atom is called as valence shell and the electrons are called as valence shell electrons. These valence shell electrons take part in all chemical reactions and are also involved in chemical bonding.
(e)
Answer to Problem 6E
Since both C and Si atoms have same number of valence electrons, therefore, they exhibit similar properties.
Explanation of Solution
Both carbon and silicon are member of same group of periodic table that is 14th group. The atomic number of carbon is 6 and the atomic number of silicon is 14. There are 4 electrons in the valence shell of both C and Si atom as shown in the shell model. Since both C and Si atoms have same number of valence electrons, therefore, they exhibit similar properties because valence electrons always take part in chemical properties and bonding of atoms.
Chapter U1 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Biology in Focus (2nd Edition)
Cosmic Perspective Fundamentals
Applications and Investigations in Earth Science (9th Edition)
Campbell Biology (11th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- For the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically: 1:1 (one mole of EDTA per mole of metal ion) 2:1 (two moles of EDTA per mole of metal ion) 1:2 (one mole of EDTA per two moles of metal ion) None of the abovearrow_forwardPlease help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forward
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





