
(a)
Interpretation:
Lewis dot structure of the given
Concept introduction:
Lewis dot structure is the representation of molecule in which valence electrons are shown as dots.
Answer to Problem 4E
Lewis structure of the given carboxylic is shown below.
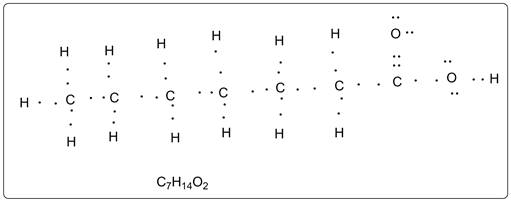
Explanation of Solution
Each carbon has four valencies. The oxygen atoms has valency two. There are two lone pairs on each O atom. Each atom is octet fulfilled except hydrogen atoms which are duplet (having 2 electrons).
(b)
Interpretation:
Structural formula of the given carboxylic acid must be drawn.
Concept introduction:
Structural formula is the representation of a molecule in which the arrangements of all the atoms are shown.
Answer to Problem 4E
Structural formula of the given carboxylic acid (heptanoic acid) is shown below.
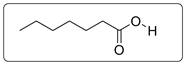
Explanation of Solution
Structural formula of heptanoic acid is drawn in which all the carbon atoms have 4 bonds. Each O has two bonds and two lone pairs of electrons. There is no lone pair on C and H atoms. Hydrogen atoms of the carbon chain are not shown to avoid complexity of the structure.
(c)
Interpretation:
The functional group of the given molecule must be predicted.
Concept introduction:
Answer to Problem 4E
There is carboxylic acid functional group in the given molecule.
Explanation of Solution
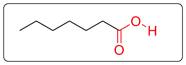
The functional group (COOH) present in heptanoic acid is shown in red. In this, one carbon is double bonded with one O and single bonded with another OH group.
(d)
Interpretation:
The smell and name of the given molecule must be predicted.
Concept introduction:
Organic molecule can be named easily following the IUPAC (International Union of Pure and applied Chemistry) nomenclature system. Trivial name is also there for organic molecule. Smell sometimes depends on the class of the molecule.
Answer to Problem 4E
Name of the molecule is heptanoic acid as per IUPAC system. Trivial name is enanthic acid. It has smell of rancid oil.
Explanation of Solution
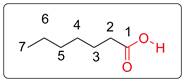
Numbering of the carbon chain is done by giving priority (number 1) to the functional group (COOH) bearing C. Total carbon is 7. So the IUPAC name is heptanoic acid. It is named as enanthic acid in trivial naming system.
The smells of this compound is like rancid oil.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Human Biology: Concepts and Current Issues (8th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Cosmic Perspective Fundamentals
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
Introductory Chemistry (6th Edition)
- For the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically: 1:1 (one mole of EDTA per mole of metal ion) 2:1 (two moles of EDTA per mole of metal ion) 1:2 (one mole of EDTA per two moles of metal ion) None of the abovearrow_forwardPlease help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forward
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





