
Concept explainers
(a)
Interpretation :
Shape of ammonia molecule must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Ammonia molecule has total 4 atoms in which N is the central atom.
(a)
Answer to Problem SII2RE
Shape of ammonia molecule is trigonal pyramidal.
Explanation of Solution
The structure of ammonia molecule is represented as follows:

N has total 4 pairs of electrons in the valence shell.
There are three bond pairs and lone pairs of electrons. N atom is sp3 hybridized and thus NH3 has trigonal pyramidal shape due to presence of one lone pair electrons.
b)
Interpretation :
Shape of silicon tetrachloride must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Silicon tetrachloride molecule has total 5 atoms in which Si is the central atom.
b)
Answer to Problem SII2RE
Shape of silicon tetrachloride molecule is tetrahedral.
Explanation of Solution
The structure is represented as follows:
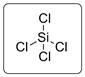
Si has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. Si atom is sp3 hybridized and thus SiCl4 has tetrahedral shape.
c)
Interpretation :
Shape of hydrogen sulfide must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Hydrogen sulfide molecule has total 3 atoms in which S is the central atom.
c)
Answer to Problem SII2RE
Shape of hydrogen molecule is angular.
Explanation of Solution
The structure of H2S is represented as follows:
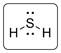
S has total 4 pairs of electrons in the valence shell.
There are two bond pairs and two lone pairs of electrons. S atom is sp3 hybridized and thus H2S has angular shape.
d)
Interpretation :
Shape of hydrogen cyanide must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Hydrogen cyanide molecule has total 3 atoms in which C is the central atom.
d)
Answer to Problem SII2RE
Shape of hydrogen cyanide is linear.
Explanation of Solution
The structure is represented as follows:
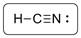
C has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. C atom is triple bonded with N and carbon is sp hybridized and thus HCN has linear shape.
e)
Interpretation :
Shape of formaldehyde must be predicted.
Concept Introduction :
Shape of a molecule is determined by the total number of electron pairs in the valence shell of the central atom.
Formaldehyde molecule has total 4 atoms in which C is the central atom.
e)
Answer to Problem SII2RE
Shape of formaldehyde is trigonal planar.
Explanation of Solution
The structure of formaldehyde is represented as follows:
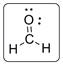
C has total 4 pairs of electrons in the valence shell.
There are four bond pairs and no lone pairs of electrons. C atom is double bonded with O and single bonded with two hydrogen atoms. Carbon is sp2 hybridized and thus CH2N has trigonal planar shape.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Introductory Chemistry (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
Campbell Biology in Focus (2nd Edition)
Microbiology with Diseases by Body System (5th Edition)
Organic Chemistry (8th Edition)
- Briefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forwardThermodynamic analysis of electrified interfaces.arrow_forward
- What is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forward
- a. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





