
Concept explainers
(a)
Interpretation:
Lewis dot structure of CF4 must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule with the valence electrons shown as dots.
(a)
Answer to Problem 5E
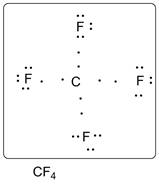
Lewis dot structure of CF4 is given below.
. 
Explanation of Solution
C has 4 valence electrons. F has 7 valence electrons. Thus one carbon is covalently bonded with 4 F atoms. There are three lone pairs of electrons on each F atom. Accordingly Lewis structure is shown as follows:
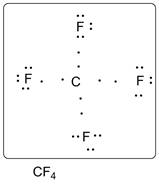
(b)
Interpretation:
Lewis dot structure of CH3Cl must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule with the valence electrons shown as dots.
(b)
Answer to Problem 5E
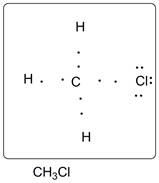
Lewis dot structure of CH3Cl is given below.
. 
Explanation of Solution
C has 4 valence electrons. Cl has 7 valence electrons. H has one valence electron. Thus one carbon is covalently bonded with 3 H atoms and 1 Cl. There are three lone pairs of electrons on Cl atom. Accordingly Lewis structure is shown as follows:
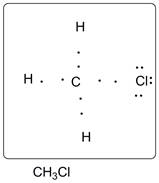
(c)
Interpretation:
Lewis dot structure of SiCl2H2 must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule with the valence electrons shown as dots.
(c)
Answer to Problem 5E
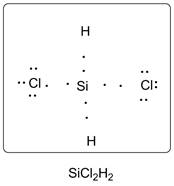
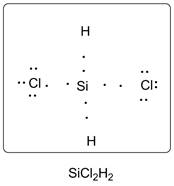
Lewis dot structure of SiCl2H2 is given below.
.
Explanation of Solution
Si has 4 valence electrons, Cl has 7 valence electrons and H has one valence electron. Thus one carbon is covalently bonded with 2 H atoms and 2 Cl atoms. There are three lone pairs of electrons on Cl atom. Accordingly Lewis structure is shown as follows:
(d)
Interpretation:
Lewis dot structure of CH3OH must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule with the valence electrons shown as dots.
(d)
Answer to Problem 5E
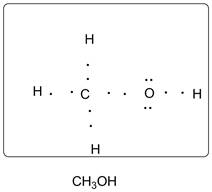
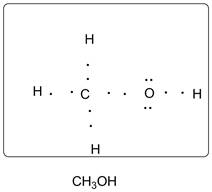
Lewis dot structure of CH3OH is given below:
.
Explanation of Solution
C has 4 valence electrons. O has 6 valence electrons. H has one valence electron. Thus one carbon is covalently bonded with 3 H atoms and 1 O atom. The O atom is bonded with central carbon atom and one hydrogen atom. There are 2 lone pairs of electrons on O atom. Accordingly, Lewis structure is shown as follows:
(e)
Interpretation:
Lewis dot structure of HOCl must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule with the valence electrons shown as dots.
(e)
Answer to Problem 5E
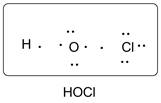
Lewis dot structure of HOCl is given below.
.
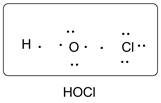
Explanation of Solution
O has 6 valence electrons, Cl has 7valence electrons and H has one valence electron. Thus one central O is covalently bonded with 1 H atoms and 1 Cl atom. There are 2 lone pairs of electrons on O atom and 3 lone pairs of electrons on Cl atom. Accordingly Lewis structure is shown as follows
(f)
Interpretation:
Lewis dot structure of CH3NH2 must be drawn.
Concept Introduction :
Lewis dot structure is the representation of a molecule with the valence electrons shown as dots.
(f)
Answer to Problem 5E
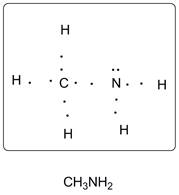
Lewis dot structure of CH3NH2 is given below.
.
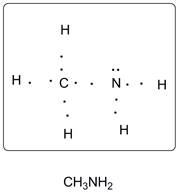
Explanation of Solution
C has 4 valence electrons, N has 5 valence electrons and H has one valence electron. Thus one central C is covalently bonded with 3 H atoms and 1 N atom. N atom is covalently bonded with central C atom and two hydrogen atoms. There are 2 lone pairs of electrons on N atom. Accordingly Lewis structure is shown as follows:
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Applications and Investigations in Earth Science (9th Edition)
Introductory Chemistry (6th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Biology: Life on Earth (11th Edition)
Microbiology: An Introduction
- Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forwardWhat is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forward
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





