
Concept explainers
Interpretation:
Whether each structural formula given is correct as per HONC 1234 rule must be explained. The incorrect structures needs to be corrected.
Concept introduction:
HONC 1234 rule states that in most of the molecule H, O, N and C generally makes 1, 2, 3 and 4 bonds respectively.
Answer to Problem 4E
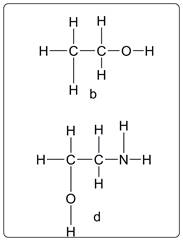
Compound b and d have violated the HONC 1234 rule.
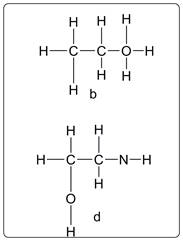
Explanation of Solution
As per the HONC 1234 rule each oxygen (O) atom must form 2 bonds and each nitrogen (N) atom must form three bonds.
As per the structure of b, O has four bonds instead of 2. Thus this is a violation of the rule. Similarly in structure d, N has two bonds instead of three. So it is violation of the rule.
The corrected structures which follow HONC 1234 rule are shown below.
Correct structures are shown as per HONC 1234 rule according to which number of bonds formed by H, O, N and C are 1, 2, 3 and 4 respectively.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Introductory Chemistry (6th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Chemistry: Structure and Properties (2nd Edition)
Chemistry: A Molecular Approach (4th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Macmillan Learning Draw the major, neutral organic product for each substitution reaction. For this question, assume that each substitution reaction goes to completion. Disregard elimination. Reaction A. CI H₂O Select Draw Templates More Erase C Harrow_forwardMacmillan Learning Reaction B: CI HO_ 곳으 / Select Draw Templates More с € H D Erasearrow_forwardWhen 2-bromo-93-dimethylbutane is heated with sodium methoxide, one majors.. në la formed. 4th attempt Part 1 (0.5 point) t Ji See Periodic Table See Hint Draw the major alkene product and all other byproducts. Be sure to include lone-pair electrons and charges. Part 2 (0.5 point) What type of mechanism is occuring? Choose one: AS1 3rd attempt X H 41 See Hint Part 1 (0.5 point) Feedback See Periodic Table See Hintarrow_forward
- Complete the mechanism for the E1 reaction below by following the directions written above each of the five boxes. Be sure to include lone pair electrons and nonzero formal charges. 2nd attempt 1 Provide the missing curved arrow notation. E+ RUDDA 1st attempt Feedback See Periodic Table See Hint Iir See Periodic Table See Hintarrow_forwardHeating an alcohol in the presence of sulfuric or phosphoric acid will cause a dehydration to occur: the removal of the elements of water from a molecule, forming an alkene. The reaction usually follows an E1 mechanism. The SN1 pathway is suppressed by using a strong acid whose conjugate base is a poor nucleophile. Further, heating the reaction mixture causes a greater increase in the rate of E1 compared to the rate of Sy1. 3rd attempt h Draw curved arrow(s) to show how the alcohol reacts with phosphoric acid. TH © 1 0 0 +1% # 2nd attempt Feedback H Ju See Periodic Table See Hint H Jud See Periodic Table See Hintarrow_forwardPart 2 (0.5 point) 0- Draw the major organic product with the correct geometry. 10 1: 70000 х く 1st attempt Part 1 (0.5 point) Feedback Please draw all four bonds at chiral centers. P See Periodic Table See Hintarrow_forward
- Heating an alcohol in the presence of sulfuric or phosphoric acid will cause a dehydration to occur: the removal of the elements of water from a molecule, forming an alkene. The reaction usually follows an E1 mechanism. The SN1 pathway is suppressed by using a strong acid whose conjugate base is a poor nucleophile. Further, heating the reaction mixture causes a greater increase in the rate of E1 compared to the rate of S№1. 2nd attempt 0 See Periodic Table See Hint Draw the organic intermediate from the first step (no byproducts) and draw curved arrow(s) to show how it reacts. TH +11: 1st attempt Feedback H H H C F F See Periodic Table See Hintarrow_forwardThis molecule undergoes an E1 mechanism when stirred in methanol. 3rd attempt CH₂OH CH₂OH 6148 O See Periodic Table. See Hint Draw 3 chemical species including formal charges and lone pairs of electrons. Add the missing curved arrow notation. H N O O SA 3 Br Iarrow_forwardComplete the mechanism for the E1 reaction below by following the directions written above each of the five boxes. Be sure to include lone pair electrons and nonzero formal charges. 1st attempt Y 0 + Provide the missing curved arrow notation. 01: See Periodic Table See Hint H C Br Iarrow_forward
- Please help answer number 2. Thanks in advance.arrow_forwardHow do I explain this? Thank you!arrow_forwardWhen an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M* = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm. The 13C NMR and DEPT spectra are provided. Draw the structure of the product as the resonance contributor lacking any formal charges. 13C NMR DEPT 90 200 160 120 80 40 0 200 160 120 80 40 0 DEPT 135 T 200 160 120 80 40 0 Draw the unknown amide. Select Dow Templates More Fragearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





