
Concept explainers
(a)
Interpretation :
Structural formula of the polar molecule with molecular formula C3H8O must be drawn.
Concept Introduction :
Structural formula represents the structure of a compound in which the arrangements of atoms are shown.
(a)
Answer to Problem 6RE
Structural formula is drawn below for the polar molecule with molecular formula C3H8O.
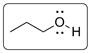
Explanation of Solution
The molecular formula C3H8O represents isomeric ether and alcohols. Alcohols are polar than ether. Two alcohols are possible with the formula. One is primary alcohol and another is secondary alcohol. Primary alcohol (Propan-1-ol) is drawn.
b)
Interpretation :
Lewis structure must be drawn and shared electrons and lone pair electrons must be labeled.
Concept Introduction :
Lewis dot structure is the representation of molecule in which valence electrons are shown as dots.
b)
Answer to Problem 6RE
Lewis dot structure is given below.
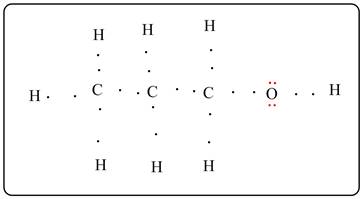
All the dots are bond pair electrons except the two electron pairs on O atom (shown in red) are lone pair electrons.
Explanation of Solution
Lewis electron dot structure is shown. All the carbon is bonded with 4 atoms and the oxygen atom is bonded with two atoms. There are all single bonds. Two lone pairs are there on O atom.
c)
Interpretation :
The dipoles must be identified.
Concept Introduction :
A molecule will have dipoles if there is electronegativity difference between the two atoms forming the bond.
c)
Answer to Problem 6RE
The dipoles are shown.
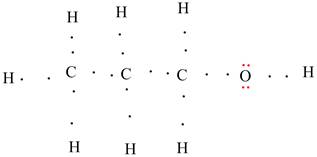
Explanation of Solution
Oxygen will have partial negative charge as it has more electronegativity. Similarly H atom will have partial positive charge as it has less electronegativity.
Each C-H bond is also a dipole in which C is the negative pole and H is the positive pole as C has more electronegativity as compared to H.
d)
Interpretation :
The geometric shape around each carbon atom must be named.
Concept Introduction :
The geometric shape around an atom depends on the number of total electron pairs.
d)
Answer to Problem 6RE
Each carbon has tetrahedral geometry.
Explanation of Solution
Each carbon is bonded with total 4 atoms including hydrogen atom, carbon atom and oxygen atom. There is no lone pair of electrons on any carbon atom. Thus, each carbon will be tetrahedral.
e)
Interpretation :
The name of the compound must be given with proper reasoning.
Concept Introduction :
A molecule can be named using IUPAC rule. The molecule will have a trivial name also.
The molecule has three carbons and -OH as a
e)
Answer to Problem 6RE
The name of the molecule is Propan-1-ol.
Explanation of Solution
The structure is given as follows:
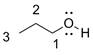
The numbering is done giving priority to the carbon bearing the functional (-OH) group. Total three carbons are there. The functional group is at carbon 1. So, the IUPAC name is propn-1-ol. The trivial name is n-propyl alcohol. As the -OH group is attached with normal propyl (1 degree) group.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Essential Biology (7th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Microbiology: An Introduction
Anatomy & Physiology (6th Edition)
Campbell Biology (11th Edition)
Biology: Life on Earth with Physiology (11th Edition)
- presented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forward
- Name the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forward
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





