
Concept explainers
(a)
Interpretation:
The name and functional group in given molecule needs to be written.
Concept Introduction :
Functional groups are moieties or specific substituents in molecules in
(a)
Answer to Problem 10E
Explanation of Solution
The seven main functional groups are:
- Hydroxyl
- Sulfhydryl
- Carbonyl
- Carboxyl
- Amino and phosphate group
- Alcohols and thiols.
The given compound is as follows:
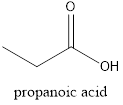
The name of the given compound is propanoic acid, because it contains a chain of three carbon atom with a function group carboxylic acid and it is a saturated compound.
(b)
Interpretation:
The name and functional group in given molecule needs to be written.
Concept Introduction :
Functional groups are moieties or specific substituents in molecules in organic chemistry, which are accountable for distinguishing chemical reactions of the given molecules.
(b)
Answer to Problem 10E
Explanation of Solution
The seven main functional groups are:
- Hydroxyl
- Sulfhydryl
- Carbonyl
- Carboxyl
- Amino and phosphate group
- Alcohols and thiols.
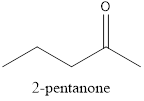
The name of the given compound is 2-pentanone, because it contains a chain of five carbon atom with a function group ketone and it is a saturated compound.
(c)
Interpretation:
The name and functional group in given molecule needs to be written.
Concept Introduction :
Functional groups are moieties or specific substituents in molecules in organic chemistry, which are accountable for distinguishing chemical reactions of the given molecules.
(c)
Answer to Problem 10E
Ketone
Explanation of Solution
The seven main functional groups are:
- Hydroxyl
- Sulfhydryl
- Carbonyl
- Carboxyl
- Amino and phosphate group
- Alcohols and thiols.
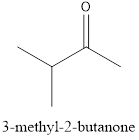
The name of the given compound is 3-methyl-2-butanone, because it contains a straight chain of four carbon atom having a branched methyl group at carbon 3 with a function group ketone and it is a saturated compound.
(d)
Interpretation:
The name and functional group in given molecule needs to be written.
Concept Introduction :
Functional groups are moieties or specific substituents in molecules in organic chemistry, which are accountable for distinguishing chemical reactions of the given molecules.
(d)
Answer to Problem 10E
Ester
Explanation of Solution
The seven main functional groups are:
- Hydroxyl
- Sulfhydryl
- Carbonyl
- Carboxyl
- Amino and phosphate group
- Alcohols and thiols.
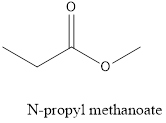
The name of the given compound is N-propyl methanoate, because it contains the functional group ester.
(e)
Interpretation:
The name and functional group in each molecule, needs to be written.
Concept Introduction :
Functional groups are moieties or specific substituents in molecules in organic chemistry, which are accountable for distinguishing chemical reactions of the given molecules.
(e)
Answer to Problem 10E
Explanation of Solution
The seven main functional groups are:
- Hydroxyl
- Sulfhydryl
- Carbonyl
- Carboxyl
- Amino and phosphate group
- Alcohols and thiols.
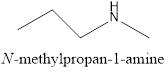
The name of the given compound is N-methylpropan-1-amine, because it contains the functional group amine.
(f)
Interpretation:
The name and functional group in each molecule, needs to be written.
Concept Introduction :
Functional groups are moieties or specific substituents in molecules in organic chemistry, which are accountable for distinguishing chemical reactions of the given molecules.
(f)
Answer to Problem 10E
Ester
Explanation of Solution
The seven main functional groups are:
- Hydroxyl
- Sulfhydryl
- Carbonyl
- Carboxyl
- Amino and phosphate group
- Alcohols and thiols.

The name of the given compound is methyl formate because it contains the functional group ester.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Biology: Life on Earth with Physiology (11th Edition)
Campbell Biology (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Anatomy & Physiology (6th Edition)
Microbiology with Diseases by Body System (5th Edition)
Campbell Essential Biology with Physiology (5th Edition)
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





