
Concept explainers
(a)
Interpretation :
HONC 1234 rules must be followed to draw the structures of the given molecular formula C3H8O2.
Concept Introduction :
HONC 1234 rule is the basic rule for drawing organic molecule. The rule states that H, O, N and C generally form 1, 2, 3 and 4 bonds respectively.
(a)
Explanation of Solution
Structures possible for C3H8O2 are given below.
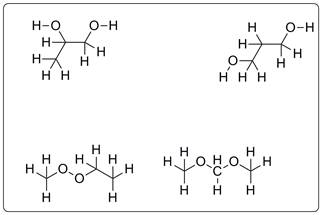
All the compounds are drawn for C3H8O2 which represents alcohols with two hydroxyl groups, peroxide and two ether groups.
b)
Interpretation :
HONC 1234 rules must be followed to draw the structures of the given molecular formula C4H11N
Concept Introduction :
HONC 1234 rule is the basic rule for drawing organic molecule. The rule states that H, O, N and C generally form 1, 2, 3 and 4 bonds respectively.
b)
Explanation of Solution
Structures possible for C4H11N are given below.
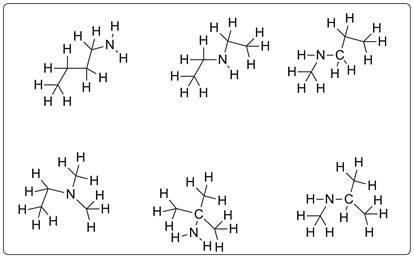
All the compounds are drawn for C4H11N which represents
c)
Interpretation :
HONC 1234 rules must be followed to draw the structures of the given molecular formula C4H10
Concept Introduction :
HONC 1234 rule is the basic rule for drawing organic molecule. The rule states that H, O, N and C generally form 1, 2, 3 and 4 bonds respectively.
c)
Explanation of Solution
Structures possible for C4H10 are given below.
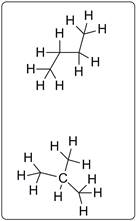
All the compounds are drawn for C4H10 which represents
d)
Interpretation :
HONC 1234 rules must be followed to draw the structures of the given molecular formula C5H12O2.
Concept Introduction :
HONC 1234 rule is the basic rule for drawing organic molecule. The rule states that H, O, N and C generally form 1, 2, 3 and 4 bonds respectively.
d)
Explanation of Solution
Structures possible for C5H12O are given below.
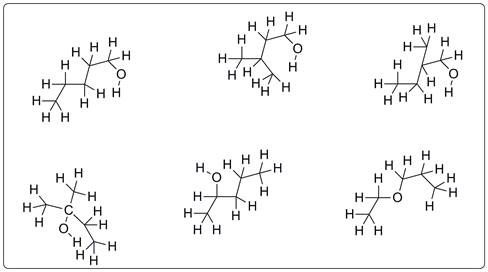
Few of the compounds for C5H12O are drawn which represents alcohols and ethers.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Introductory Chemistry (6th Edition)
College Physics: A Strategic Approach (3rd Edition)
Campbell Essential Biology (7th Edition)
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





