
Concept explainers
(a)
Interpretation:
Lewis dot structure of butane must be drawn.
Concept Introduction :
Butane has molecular formula C4H10.
Lewis dot structure is the representation of a molecule where all the valence electrons of participating atoms are shown as dots.
(a)
Answer to Problem 7E
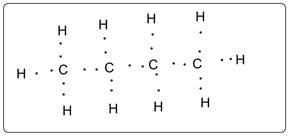
Lewis dot structure Butane is shown below.
Explanation of Solution
Lewis dot structure of butane is shown in which all the C is tetravalent. There is no lone pair of electron. All the C is octet fulfilled whereas all the hydrogens are duplet.
(b)
Interpretation:
The number of electron domains there in butane must be explained.
Concept Introduction :
Electron domain is the regions of molecules where there are bond pairs and lone pairs of electrons. Each bond between two participating atoms has electron domains.
(b)
Answer to Problem 7E
Butane has 13 electron domains.
Explanation of Solution
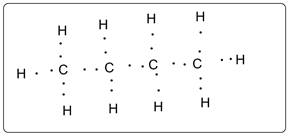
Structure of butane is shown. Each C has 4 bonds and each H has 1 bond. Thus there are total 13 bonds which are electron domains.
(c)
Interpretation:
Shape of butane must be predicted.
Concept Introduction :
Shape of a molecule depends on the total number of electrons pairs including bond pairs and lone pairs on an atom.
(c)
Answer to Problem 7E
Butane has zigzag shape in which all the carbon atoms have tetrahedral geometry.
Explanation of Solution
Each carbon atom has total 4 bonds. Each carbon atom is bonded to either 2 or 3 hydrogen atoms and 1 or 2 other carbon atoms. Thus the shape of the molecule is zigzag with tetrahedral arrangements of bonds around each C atom.
(d)
Interpretation:
The reason for the carbon atom chain to be not straight must be explained.
Concept Introduction :
A molecule attains a shape in which repulsive interaction among bonds is minimum.
(d)
Answer to Problem 7E
Carbon atom chain in butane is not straight to stabilize the molecule.
Explanation of Solution
Each carbon atom has four bonds which are tetrahedrally arranged to give maximum stability to the molecule.
Thus butane doesn’t have straight chain structure.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology with Diseases by Body System (5th Edition)
Microbiology: An Introduction
Organic Chemistry (8th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- The quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardIf the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forward
- When propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forwardDoes Avogadro's number have units?arrow_forward
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





