
(a)
Interpretation:
Lewis dot structure of the given
Concept introduction:
Lewis dot structure is the representation of molecule in which valence electrons are shown as dots.
Answer to Problem 6E
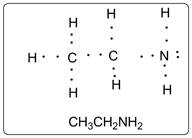
Lewis structure of the given amine is shown below.
Explanation of Solution
Each carbon has four valencies. The nitrogen atom has valency three. There is one lone pair on N atom. All the atoms have single bond. Each atom is octet fulfilled except hydrogen atoms which are duplet (having 2 electrons).
(b)
Interpretation:
Structural formula of the given amine must be drawn.
Concept introduction:
Structural formula is the representation of a molecule in which the arrangements of all the atoms are shown.
Answer to Problem 6E
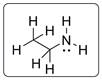
Structural formula of the given amine (ethanamine) is shown below.
Explanation of Solution
Structural formula of ethanamine is drawn in which the two carbon atoms are bonded with four atoms and the nitrogen atom is bonded with two hydrogen atoms and one carbon atoms. Additionally, there is one lone pair electrons on N atom. There is no lone pair on C and H atoms.
(c)
Interpretation:
The functional group of the given molecule must be predicted.
Concept introduction:
Answer to Problem 6E
There is amine functional group in the given molecule.
Explanation of Solution
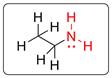
The functional group (NH2) present in ethanamine is shown in red. It is a primary amine functional group in which the N atom is bonded with one carbon atom and two hydrogen atoms. There is also one lone pair on N atom.
(d)
Interpretation:
The smell and name of the given molecule must be predicted.
Concept introduction:
Organic molecule can be named easily following the IUPAC (International Union of Pure and applied Chemistry) nomenclature system. Trivial name is also there for organic molecule. Smell sometimes depends on the class of the molecule.
Answer to Problem 6E
Name of the molecule is ethanamine as per IUPAC system. Trivial name is ethyl amine. It has pungent smell like ammonia.
Explanation of Solution
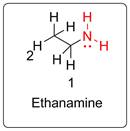
Numbering of the carbon chain is done giving priority (number 1) to the functional group bearing C. Total carbon is 2. So the IUPAC name is ethanamine. As there is ethyl (C2H5) group attached with the amine functional group, thus it is named as ethyl amine in trivial naming system.
The smells of most of the amines are like ammonia as these are ammonia derivatives. Thus, smell of ethanamine is pungent like ammonia.
Chapter U2 Solutions
Living By Chemistry: First Edition Textbook
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Biology: Life on Earth (11th Edition)
Campbell Essential Biology (7th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Introductory Chemistry (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
- Draw a stepwise mechanism for the following reaction. OHarrow_forwardHelp with annotating the labeled peaks in the 'H NMR (solvent CDCls) spectra and 'H NMR (solvent Acetone-D6) spectra Also help with Calculating the keto-enol tautomerization Ka constant for the product in both solvents.Two solvents and two different Kaarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide CH₂OH HO H HO H H OH CH₂OHarrow_forward
- Can you explain how I get these here and show the steps plz?arrow_forwardGive the IUPAC name for this compound Hydrocarbon Condensed Formulas Hint C2H5 CH2CH3 expand that in all the formula Part A: (CH3)2CHCH(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part B: CH2=C(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part C: (CH3)2C=CHC(C2H5)=CH2 Give the IUPAC name for this compound. Part D: CH3C=CCH(C2H5)2 Give the IUPAC name for this compound. Part E: (CH3)3CC=CCH2CH=C(CH3)2arrow_forwardSelect/ Match the correct letter from the image below for the IUPAC names given below: A B C D 3 E F G H K L Part 1. 4-methylheptane For example.mmmm Answer Letter H _for part 1 Part 2. 2,4-dimethylhexane Part 3. 2,3-dimethylpentane Part 4. 2,2-dimethylhexane Part 5. 2-ethyl-1,1,3,3-tetramethylcyclopentane Part 6. 3-ethyl-2-methylpentanearrow_forward
- Can u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forwardUse IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forward
- Q5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forwardFind chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





