
(a)
Interpretation: The molecular formulas for citronellol and geraniol needs to be determined.
Concept Introduction: Chemical compounds with same molecular formula having different structure are known as isomers. Isomers having same number of atoms of each type but bonded together in a different sequence (spatial arrangement) are known as stereoisomers.
There are two kinds of stereo isomers- Geometrical and optical.
(a)
Explanation of Solution
The given structures are as follows:
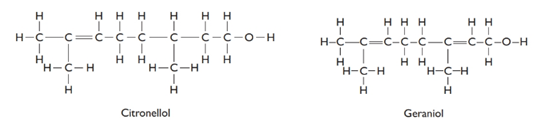
From the above structures, molecular formula of the compounds can be determined as follows:
C10H21O −Citronellol
C10H18O- Gerniol
(b)
Interpretation: The two distinct smells of the molecule citronellol needs to be explained.
Concept Introduction: Chemical compounds with same molecular formula having different structure are known as isomers. Isomers having same number of atoms of each type but bonded together in a different sequence (spatial arrangement) are known as stereoisomers.
There are two kinds of stereo isomers- Geometrical and optical.
(b)
Explanation of Solution
The formula of citronellol is C10H21O. The structure is represented as follows:
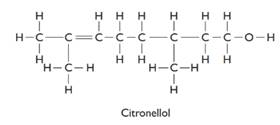
There are two optical isomers which are non-superimposable mirror image of each other.
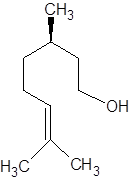
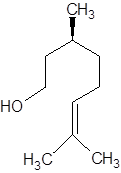
Isomer of Citronellol
Thus, the two different smells are due to two different optical isomers of citronellol.
(c)
Interpretation: The reason for only one smell of geraniol smell needs to be explained.
Concept Introduction: Chemical compounds with same molecular formula having different structure are known as isomers. Isomers having same number of atoms of each type but bonded together in a different sequence (spatial arrangement) are known as stereoisomers.
There are two kinds of stereo isomers- Geometrical and optical.
(c)
Explanation of Solution
The molecular formula of geraniol is C10H18O. The structure is represented as follows:
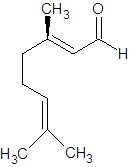
Geraniol
It can show geometrical isomerism. The E-isomer and Z-isomer are as follows:
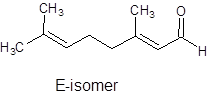
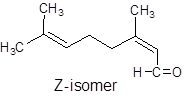
Trans-Form Cis-Form
Due to absence of optical isomerism, there is only one smell of the molecule.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Human Anatomy & Physiology (2nd Edition)
Introductory Chemistry (6th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Biology (11th Edition)
Microbiology: An Introduction
- What is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forwardΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forward
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





