
Concept explainers
(a)
Interpretation: Molecular formula needs to be determined for
Concept introduction: Atoms combine to form molecules. Molecular formula is the chemical formula, which shows actual number of atoms of each element present in the molecule of the compound.
(a)
Answer to Problem 5E
Explanation of Solution
The structure of the molecule is as follows:
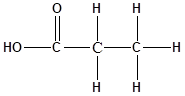
Total number of ‘C’ atom = 3
Total number ‘H’ atom = 6
Total number of ‘O’ atom = 2
Therefore the molecular formula is
(b)
Interpretation: The molecular formula needs to be determined for
Concept introduction: Atoms combine to form molecules. Molecular formula is the chemical formula, which shows actual number of atoms of each element present in the molecule of the compound.
(b)
Answer to Problem 5E
Explanation of Solution
The structure of the molecule is represented as follows:
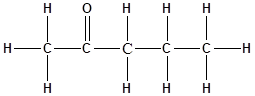
Total number of ‘C’ atom = 5
Total number ‘H’ atom = 10
Total number of ‘O’ atom = 1
Therefore, the molecular formula is
(c)
Interpretation: Molecular formula needs to be determined for the compound
Concept introduction: Atoms combine to form molecules. Molecular formula is the chemical formula, which shows actual number of atoms of each element present in the molecule of the compound.
(c)
Answer to Problem 5E
Explanation of Solution
The structure of the molecule is as follows:
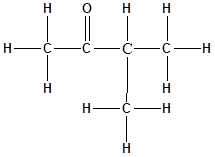
Total number of ‘C’ atom = 5
Total number ‘H’ atom = 10
Total number of ‘O’ atom = 1
Therefore the molecular formula is
(d)
Interpretation: Molecular formula needs to be determined for the compound
Concept introduction: Atoms combine to form molecules. Molecular formula is the chemical formula, which shows actual number of atoms of each element present in the molecule of the compound.
(d)
Answer to Problem 5E
Explanation of Solution
The structure of the molecule is as follows:
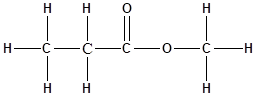
Total number of ‘C’ atom = 4
Total number ‘H’ atom = 8
Total number of ‘O’ atom = 2
Therefore, the molecular formula is
(e)
Interpretation: Molecular formula needs to be determined for the compound
Concept introduction: Atoms combine to form molecules. Molecular formula is the chemical formula, which shows actual number of atoms of each element present in the molecule of the compound.
(e)
Answer to Problem 5E
Explanation of Solution
The structure of the molecule is as follows:
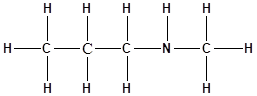
Total number of ‘C’ atom = 4
Total number ‘H’ atom = 11
Total number of ‘N’ atom = 1
Therefore, the molecular formula is
(f)
Interpretation: The molecular formula needs to be determined in the compound
Concept introduction: Atoms combine to form molecules. Molecular formula is the chemical formula, which shows actual number of atoms of each element present in the molecule of the compound.
(f)
Answer to Problem 5E
Explanation of Solution
The structure of the molecule is as follows:
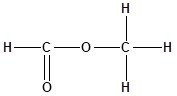
Total number of ‘C’ atom = 4
Total number ‘H’ atom = 4
Total number of ‘O’ atom = 2
Therefore, the molecular formula is
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Anatomy & Physiology (6th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Microbiology: An Introduction
Human Physiology: An Integrated Approach (8th Edition)
- Hint These are benzene substitution reactions. ALCI3 and UV light are catalyst no part in reactions and triangle A means heating. A. Add ethyl for Et in benzene ring alkylation reaction EtCl = CH3CH2CL 1) EtC1 / AlCl3 / A ? B: Add Br to benzene ring ( substitution) 2) Br₂ / uv light ? C Add (CH3)2 CHCH2 in benzene ring ( substitution) (CH3)2CHCH,C1 / AICI, ?arrow_forwardDraw the mechanism to make the alcohol 2-hexanol. Draw the Mechanism to make the alcohol 1-hexanol.arrow_forwardDraw the mechanism for the formation of diol by starting with 1-pentanal in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
- Draw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardDraw the mechanism for the oxidation of 3-bromo-cyclohexan-1-ol.arrow_forwardConvert the following Fischer projection to Haworth projections. show work and show the arrows please.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





