
(a)
Interpretation:
Lewis dot structure must be drawn for methyl acetate.
Concept introduction:
A Lewis dot structure is the representation of the molecule in which all the valence electrons are used and shown as dots.
Answer to Problem 5E
Lewis dot structure of methyl acetate is given below:
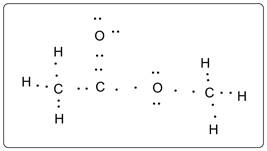
Explanation of Solution
Each carbon atom has formed 4 bonds. Each O atom has formed two bonds. Each H atom has formed 1 bond. Each O atom has two lone pairs of electrons.
(b)
Interpretation:
Structural formula must be drawn for the given molecular formula C3H6O2.
Concept introduction:
Structural formula is the formula of a molecule which shows the arrangements of atoms that is how all the atoms are bonded with each other.
Answer to Problem 5E
Structural formula for C3H6O2is given below.
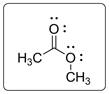
Explanation of Solution
The given molecule is an ester. The central carbon atom is bonded with carbon atom of methyl (CH3) group, O atom with a double bond and another O atom with a single bond. The two lone pairs on each O atom are shown as pair of dots.
(c)
Interpretation:
The functional group in the given molecule must be predicted.
Concept introduction:
Answer to Problem 5E
In the given molecule there is ester functional group.
Explanation of Solution
Esters are acid derivative with the formula RCOOR’. Where R and R’ are alkyl, aryl or other groups.
The functional group is shown below.
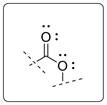
(d)
Interpretation:
The name and smell of the given compound must be predicted.
Concept introduction:
Smell of a compound depends on the structure of the molecule particularly on the functional group present.
Answer to Problem 5E
The name of the compound is methyl acetate or Methyl ethanoate.
The smell is like ripe banana.
Explanation of Solution
Esters have fruity smell. The acid counterpart of the ester is obtained from acetic acid or ethanoic acid. The alcohol counterpart is obtained from methyl alcohol or methanol. Thus it is an ester of acetic acid and methyl alcohol. So, the name is methyl acetate and IUPAC name is methyl ethanoate.
It has sweet fruity smell resembling ripe banana.
Chapter U2 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Organic Chemistry (8th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology: An Introduction
- Write all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





