
(a)
Interpretation: Balanced chemical equation for the combustion of ethane needs to be written.
Concept Introduction : Combustion is a
(a)
Answer to Problem 3E
Balanced chemical equation for the combustion of ethane is,
Explanation of Solution
The chemical formula of ethane is
The above equation is not balanced. Balanced chemical equation is the chemical equation in which number of atoms of every element is equal in reactant and product side of the chemical reaction.
To balance the equation, add coefficient 2 to
(b)
Interpretation: The energy diagram for combustion of ethane needs to be drawn.
Concept Introduction : Combustion is a chemical reaction in which a compound or an element undergoes reaction with oxygen and releases energy in the form of light and heat. Combustion of covalent compounds comprising carbon and hydrogen produces carbon dioxide and water. So, ethane undergoes combustion reaction to produce carbon dioxide and water.
(b)
Answer to Problem 3E
The energy diagram for combustion of ethane is,
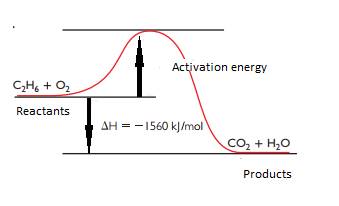
Explanation of Solution
From the chapter 103, the heat of combustion of ethane is
So, the heat energy diagram for combustion of ethane is,
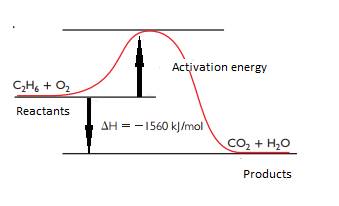
(c)
Interpretation: The net energy exchange of the reverse reaction to form ethane is to be determined.
Concept Introduction : In a chemical process, energy is conserved. This means, net exchange of energy in a forward process is equal and opposite to exchange of net energy in the reverse process. If a forward reaction exothermic, the reverse reaction is endothermic.
(c)
Answer to Problem 3E
The net energy exchange of reverse reaction to form ethane and oxygen is
Explanation of Solution
The combustion reaction of ethane is as follows:
The net energy released in the process of combustion of ethane is
Chapter U5 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Human Biology: Concepts and Current Issues (8th Edition)
Concepts of Genetics (12th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Cosmic Perspective Fundamentals
Applications and Investigations in Earth Science (9th Edition)
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forward
- 8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





