
Concept explainers
Interpretation:
The reason for colors to be described in terms of wavelength rather than words needs to be explained.
Concept introduction:
Humans can see color due to interaction between emitted and reflected light by an object and eye. The emitted or reflected light waves by an object enter the eye and passed the cornea of the eye where refraction of light takes place. The refracted light again undergoes refraction when it is passed through the transparent lens. This lens focuses the light waves on the retina where image of the object is formed.
Explanation of Solution
The object absorbs certain wavelength or
The
If an object reflects all the colors of the light, it will appear white and if it absorbs all the colors, it will appear black.
The observed light is always complementary to the color of the light absorbed by it.
The following color wheel represents the color and the wavelength range in which they are absorbed.
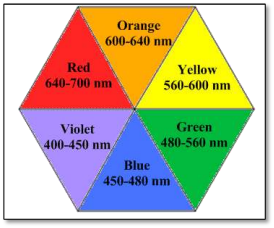
For example, from the color wheel, the color of light complementary to yellow is violet. Thus, if an object absorbs in the wavelength range of 400-450 nm which is for violet color thus, the wavelength of yellow light that is 560-600 nm is transmitted by the object. Therefore, the object appears yellow color.
Since, the color of an object depends on the wavelength of light absorbed by it thus, it is important to describe colors in terms of wavelength rather than words.
Chapter U5 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Microbiology: An Introduction
College Physics: A Strategic Approach (3rd Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Microbiology: An Introduction
Introductory Chemistry (6th Edition)
- Which of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forwardDraw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forward
- Draw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forwardWhat is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forwardIndicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





