
(a)
Interpretation:
The direction of energy transferred in the process of conversion of water to ice is to be discussed.
Concept introduction:
Whenever the state of a substance changes, a transfer of heat takes place.
Conversion of solid to liquid or liquid to gas requires heat and gas to liquid or liquid to solid releases heat.
(a)
Answer to Problem C18.4RE
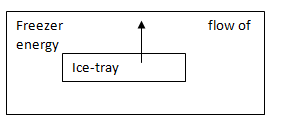
Energy is transferred from water to the freezer.
Explanation of Solution
When the water is in a liquid form in the freezer, it is converted to ice which is a solid form. A fixed amount of energy is released when liquid is converted to solid. So, there is a transfer of energy from water to the freezer.
(b)
Interpretation:
The energy transferred in the process of conversion of water to ice is whether endothermic or exothermic for ice tray is to be discussed.
Concept introduction:
Whenever the state of a substance changes, a transfer of heat takes place.
Conversion of solid to liquid or liquid to gas requires heat and gas to liquid or liquid to solid releases heat.
A process is endothermic when there is an absorption of heat and exothermic when there is a release of heat.
(b)
Answer to Problem C18.4RE
The process is exothermic for the ice tray.
Explanation of Solution
When the water is in a liquid form in the freezer, it is converted to ice which is a solid form. A fixed amount of energy is released when liquid is converted to solid. So, there is a transfer of energy from water to the freezer. So, the process is exothermic by virtue of tray.
(c)
Interpretation:
The amount of heat transferred for one ice cube is to be calculated.
Concept introduction:
Whenever the state of a substance changes, a transfer of heat takes place.
Conversion of solid to liquid or liquid to gas requires heat and gas to liquid or liquid to solid releases heat.
A process is endothermic when there is an absorption of heat and exothermic when there is a release of heat.
The amount of heat released when 1 mol of water freezes is called enthalpy of freezing.
(c)
Answer to Problem C18.4RE
Amount of heat released
Explanation of Solution
The heat released when 1 mol water freezes
Density of water=
Therefore,
Thus,heat released when
So, heat released when
(d)
Interpretation:
The amount of heat transferred for full ice tray is to be calculated.
Concept introduction:
Whenever the state of a substance changes, a transfer of heat takes place.
Conversion of solid to liquid or liquid to gas requires heat and gas to liquid or liquid to solid releases heat.
A process is endothermic when there is an absorption of heat and exothermic when there is a release of heat.
The amount of heat released when 1 mol of water freezes is called enthalpy of freezing.
(d)
Answer to Problem C18.4RE
Amount of heat released
Explanation of Solution
The heat released when 1 mol water freezes
Density of water=
Therefore,
Thus,heat released when
So, heat released when
Number of moulds in ice tray
Therefore,
Heat released when full tray is frozen
Chapter U5 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Fundamentals of Anatomy & Physiology (11th Edition)
Human Anatomy & Physiology (2nd Edition)
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
Campbell Biology (11th Edition)
Cosmic Perspective Fundamentals
- What are the major products of the following organic reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardWhat are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following organic reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forward
- Predict the organic product that forms in the reaction below: H + гон OH H+ H+ ☑ O Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the product. In the drawing area below, draw the skeletal ("line") structure of the missing organic product X. Explanation Check Click and drag to start drawing a structure. S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardIn the analysis of Mg content in a 25 mL sample, a titration volume of 5 mL was obtained using 0.01 M EDTA. Calculate the Mg content in the sample if the Ca content is 20 ppmarrow_forwardPredict the organic products that form in the reaction below: H. H+ + OH H+ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. G X C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access +arrow_forward
- 111 Carbonyl Chem Choosing reagants for a Wittig reaction What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 1 2 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure. × ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. . If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T X O O лет-ле HO OH HO OH This transformation can't be done in one step.arrow_forwardDetermine the structures of the missing organic molecules in the following reaction: X+H₂O H* H+ Y OH OH Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. X Sarrow_forward
- Predict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. HO. O :☐ + G Na O.H Click and drag to start drawing a structure. XS xs H₂Oarrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H H C H- a -H b H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal groups may have slightly different sizes. a = b = 0 °arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? :0: HCOH a Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0 b=0° Sarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





