
Concept explainers
(a)
Interpretation:
The sketches of two waves need to be drawn to show the difference between green light and blue light. The appropriate wavelengths of these two waves need to be determined.
Concept introduction:
The wavelength of a wave is defined as the distance between two consecutive crest and trough.
Explanation of Solution
The waves for short and long wavelength are represented as follows:
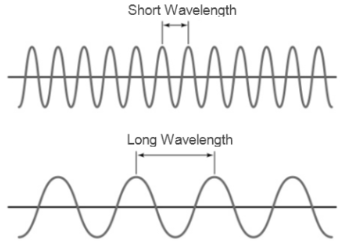
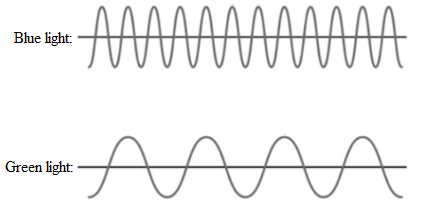
From the visible spectra, the range of wavelength for green and blue light is 560-520 nm and 490-450 nm respectively.
Thus, blue light has short wavelength than green light. The sketches of two waves will be as follows:
(b)
Interpretation:
The approximate frequencies of these two waves need to be determined.
Concept introduction:
The frequency of the wave is defined as number of waves passing a point in 1 second.
Explanation of Solution
The relation between wavelength and frequency is as follows:
Here,
Since, wavelength is inversely proportional to the
Thus, the wave with short wavelength have high frequency and wave with long wavelength have low frequency.
From the visible region, the range of frequency for green and blue light is 540 to 580 THz and 610-670 THz respectively.
(c)
Interpretation:
The speed of the travelling waves needs to be determined.
Concept introduction:
The
When this proportionality constant is removed, a constant c is used.
This c is speed of light.
Explanation of Solution
The relation between wavelength and frequency is as follows:
Here,
The speed of light is a constant with value
Chapter U5 Solutions
Living by Chemistry
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Human Physiology: An Integrated Approach (8th Edition)
Biology: Life on Earth (11th Edition)
Biology: Life on Earth with Physiology (11th Edition)
The Cosmic Perspective (8th Edition)
Applications and Investigations in Earth Science (9th Edition)
- These are in the wrong boxes. Why does the one on the left have a lower molar mass than the one on the right?arrow_forwardSYNTHESIS REACTIONS. For the following reactions, synthesize the given products from the given reactants. Multiple reactions/steps will be needed. For the one of the steps (ie reactions) in each synthesis, write out the mechanism for that reaction and draw an energy diagram showing the correct number of hills and valleys for that step's mechanism. CI b. a. Use acetylene (ethyne) and any alkyl halide as your starting materials Br C. d. "OH OH III. OHarrow_forwardCalculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.200 M HClarrow_forward
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





