
Interpretation:
The chemical formula for the polyatomic APA ion is to be written.
Concept introduction:
In the molecular formula, the element symbols and the number of the subscript tells the type and the number of each atom in a molecule.
There are so many methods to represent a molecule. In the ball-and-stick method, the atoms of each element are depicted by a ball and the bond is depicted by a stick. In the structural formula method, the atoms of each element are depicted by letter symbols and bond is used to show relative position.
Answer to Problem 145A
The chemical formula for the polyatomic APA ion is
Explanation of Solution
Ions are atoms or groups of atoms with an overall charge. The charge can be positive or negative. The structural formula for the APA ion is given in the figure shown below.
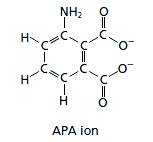
In the given structural formula, there are eight carbon atoms, five hydrogen atoms, one nitrogen atom, four oxygen atoms, and two negative charges. Therefore, the chemical formula for the polyatomic APA ion is
The chemical formula for the polyatomic APA ion is
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Biology: Life on Earth (11th Edition)
Microbiology: An Introduction
Human Anatomy & Physiology (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
Chemistry: Structure and Properties (2nd Edition)
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





