
Concept explainers
Interpretation:
The wavelength of photons emitted by the given element is to be stated.
Concept introduction:
When light passes through the prism, it separates into different colors of light with different frequencies. The pattern of these different lines is termed as atomic emission spectrum.
Answer to Problem 12STP
The wavelength of photons emitted by the given element is 580 nm.
Explanation of Solution
The atomic emission spectrum of given element is shown below.
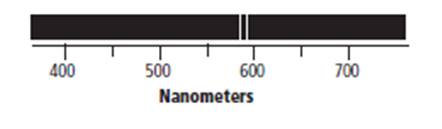
The above atomic emission spectrum of the given element shows that the first emission band appears at 580 nm. Therefore, the wavelength of photon emitted by the given element via using atomic emission spectrum is approximately 580 nm.
The wavelength of photon emitted by the given element via using atomic emission spectrum is 580 nm.
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Cosmic Perspective Fundamentals
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Concepts of Genetics (12th Edition)
Biology: Life on Earth (11th Edition)
- Part C A solution that is 0.040 M in HCIO4 and 0.046 M in HCI Express your answer numerically to two decimal places. ΜΕ ΑΣΦ ? pH = Submit Request Answer Part D A solution that is 1.08% HCl by mass (with a density of 1.01 g/mL) Express your answer numerically to three decimal places. ΜΕ ΑΣΦ -> 0 ? pH =arrow_forwardPredict the equilibrium arrows for the following reaction:*see imagearrow_forwardProvide the missing information for each of the two following reacitons: *see imagearrow_forward
- Draw an example of the following functional groups: *see imagearrow_forwardAldehydes and Ketones: Show the reaction conditions, and molecules, that connect the reactant to the product. A protecting group will be needed. *see imagearrow_forwardAldehydes and Ketones: Show the reaction conditions, and molecules, that connect the reactant to the product. *see imagearrow_forward
- Provide the missing information for each of the four reactions: *see imagearrow_forward6. Chlorine dioxide (CIO) is used as a disinfectant in municipal water-treatment plants. It decomposes in a first-order reaction with a rate constant of 14 s. How long would it take for an initial concentration of 0.06 M to decrease to 0.02 M? [6 pts]arrow_forwardIf possible, replace an H atom on the a carbon of the molecule in the drawing area with a methyl group substituent, and replace an H atom on the ẞ carbon with a hydroxyl group substituent. If one of the substituents can't be added for any reason, just don't add it. If neither substituent can be added, check the box under the drawing area. en HO OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediate and product of this hydrohalogenation reaction. Include all lone pairs and charges as appropriate. Br Select to Draw 51°F Sunny esc F1 HBr Select to Draw 1,2-hydride shift Br Select to Draw Q Search F2 F3 F4 1 2 # # 3 DII L F5 F6 F tA $ % Λarrow_forwardplease help i cant find the article to even startarrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





