
Concept explainers
Interpretation:
The sigma bond, pi bond, and lone pairs present in the urea are to be identified.
Concept introduction:
In the molecular formula, the element symbols and the subscripts number tells the type and the number of each atom in a molecule. In structural formula method, the atoms of each element are depicted by letter symbols and bond is used to show relative position.
Answer to Problem 134A
The
Explanation of Solution
The structure of acetonitrile is shown below.
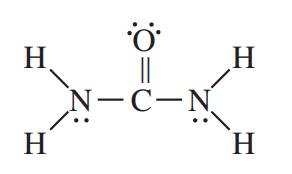
It is known that in single bond only sigma bond is present. In double bond, one bond is sigma and one is pi bond. In triple bond, one bond is sigma and the remaining two bonds are pi bond. The lone pairs are non bonding electrons represented by two dots.
Therefore, the representation of sigma bonds, pi bonds, and lone pairs are shown below.
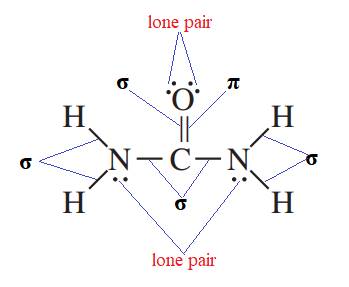
Therefore,
The
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
College Physics: A Strategic Approach (3rd Edition)
Microbiology: An Introduction
Biology: Life on Earth (11th Edition)
Brock Biology of Microorganisms (15th Edition)
Human Anatomy & Physiology (2nd Edition)
- 32. Consider a two-state system in which the low energy level is 300 J mol 1 and the higher energy level is 800 J mol 1, and the temperature is 300 K. Find the population of each level. Hint: Pay attention to your units. A. What is the partition function for this system? B. What are the populations of each level? Now instead, consider a system with energy levels of 0 J mol C. Now what is the partition function? D. And what are the populations of the two levels? E. Finally, repeat the second calculation at 500 K. and 500 J mol 1 at 300 K. F. What do you notice about the populations as you increase the temperature? At what temperature would you expect the states to have equal populations?arrow_forward30. We will derive the forms of the molecular partition functions for atoms and molecules shortly in class, but the partition function that describes the translational and rotational motion of a homonuclear diatomic molecule is given by Itrans (V,T) = = 2πmkBT h² V grot (T) 4π²IKBT h² Where h is Planck's constant and I is molecular moment of inertia. The overall partition function is qmolec Qtrans qrot. Find the energy, enthalpy, entropy, and Helmholtz free energy for the translational and rotational modes of 1 mole of oxygen molecules and 1 mole of iodine molecules at 50 K and at 300 K and with a volume of 1 m³. Here is some useful data: Moment of inertia: I2 I 7.46 x 10- 45 kg m² 2 O2 I 1.91 x 101 -46 kg m²arrow_forwardK for each reaction step. Be sure to account for all bond-breaking and bond-making steps. HI HaC Drawing Arrows! H3C OCH3 H 4 59°F Mostly sunny H CH3 HO O CH3 'C' CH3 Select to Add Arrows CH3 1 L H&C. OCH3 H H H H Select to Add Arrows Q Search Problem 30 of 20 H. H3C + :0: H CH3 CH3 20 H2C Undo Reset Done DELLarrow_forward
- Draw the principal organic product of the following reaction.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided structures, draw the curved arrows that epict the mechanistic steps for the proton transfer between a hydronium ion and a pi bond. Draw any missing organic structures in the empty boxes. Be sure to account for all lone-pairs and charges as well as bond-breaking and bond-making steps. 2 56°F Mostly cloudy F1 Drawing Arrows > Q Search F2 F3 F4 ▷11 H. H : CI: H + Undo Reset Done DELLarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbons. Draw out the benzene ring structure when doing itarrow_forward
- 1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series. 2) Calculate the ionization energy of He* and L2+ ions in their ground states. 3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.arrow_forwardCalculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forward
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseeearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





