
Concept explainers
(a)
Interpretation:
Whether
Concept introduction:
The asymmetric structure and presence of polar covalent bonds confirms that the molecule is polar in nature. The molecule having some dipole moment is termed as polar molecule and the molecule with no dipole moment or zero dipole moment is identified as nonpolar molecule.
Answer to Problem 122A
The
Explanation of Solution
The given ion
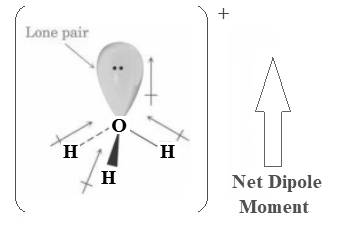
In
(b)
Interpretation:
Whether
Concept introduction:
The asymmetric structure and presence of polar covalent bonds confirms that the molecule is polar in nature. The molecule having some dipole moment is termed as polar molecule and the molecule with no dipole moment or zero dipole moment is identified as nonpolar molecule.
Answer to Problem 122A
The
Explanation of Solution
The given molecule
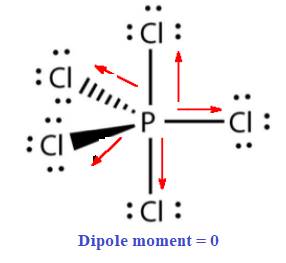
In
(c)
Interpretation:
Whether
Concept introduction:
The asymmetric structure and presence of polar covalent bonds confirms that the molecule is polar in nature. The molecule having some dipole moment is termed as polar molecule and the molecule with no dipole moment or zero dipole moment is identified as nonpolar molecule.
Answer to Problem 122A
The
Explanation of Solution
The given molecule
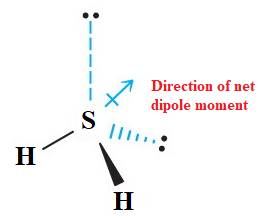
In
(d)
Interpretation:
Whether
Concept introduction:
The asymmetric structure and presence of polar covalent bonds confirms that the molecule is polar in nature. The molecule having some dipole moment is termed as polar molecule and the molecule with no dipole moment or zero dipole moment is identified as nonpolar molecule.
Answer to Problem 122A
The
Explanation of Solution
The given molecule
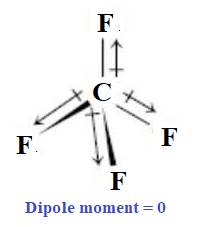
In
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Microbiology with Diseases by Body System (5th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Anatomy & Physiology (6th Edition)
Campbell Biology (11th Edition)
Campbell Biology in Focus (2nd Edition)
Introductory Chemistry (6th Edition)
- please help i cant find the article to even startarrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardhelp with the rf values i am so confusedarrow_forward
- Predict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardPredict the major organic product for this reaction.arrow_forwardPredict the major organic product for this reaction.arrow_forward
- Predict the major organic product for this reaction.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





