
Concept explainers
Interpretation:
The polar molecules need to be determined.
Concept introduction:
The bond formed between two atoms having a large electro negativity difference is polar. This is becausethe difference in electro negativity creates partial negative and positive charges on the atoms. The more electronegative atom gets a partial negative charge and a less electronegative atom gets a partial positive charge.
A covalent bond can be polar in nature if the difference in electro negativity value falls in the range of 0.6-1.9.
All the ionic bonds are polar in nature with the value of electro negativity difference more than 1.9.
Also, the overall molecule can be polar or non-polar depending on the overall dipole moment. A molecule having polar bonds can be overall non-polar if all the dipole moments get canceled out.
Answer to Problem 127A
Explanation of Solution
Given Information: The given molecules are as follows:
Reason for correct options:
The given molecule is
Here, the electro negativity of Cl is 3.16 and that of C is 2.55. The difference in electro negativity is 0.61. Thus, the C-Cl bond is polar in nature. The electro negativity difference of C and H is not that much thus, it has a non-polar covalent bond.
The Lewis structure is represented as follows:
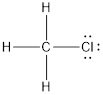
The direction of the dipole moment is towards Cl atom and it does not get canceled out thus, the overall molecule is polar in nature.
Now considering ClF,
Here, Cl-F bond isa polar bond as the electro negativity of Cl and F is 3.2 and 4 respectively.
The electronegative difference is 4-3.2 = 0.8 which is more than 0.6.
There is no other bond in the molecule. Therefore, CIF is a polar molecule.
Considering the NCl3molecule.
The Lewis structure is represented as follows:
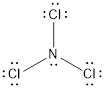
The electro negativity of N and Cl is 3.07 and 2.88 respectively. The electro negativity difference will be 0.19 which is very small thus, the N-Cl bond is not polar. But, there is one lone pair of electron on the N which makes the overall molecule polar as the electron density is more where the lone pair is present.
The direction of the dipole is towards lone pair making the molecule slightly polar in nature.
Reason for incorrect options:
Considering
The Lewis structure is represented as follows:
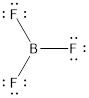
The above molecule is symmetrical and planar in nature thus, dipole moments get canceled out and the overall molecule is non-polar.
Considering
The Lewis structure is represented as follows:

The molecule is linear and both S atoms have the same number of lone pairs of electrons thus, the overall molecule is non-polar in nature.
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Microbiology: An Introduction
Concepts of Genetics (12th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Introductory Chemistry (6th Edition)
Human Physiology: An Integrated Approach (8th Edition)
College Physics: A Strategic Approach (3rd Edition)
- 43) 10.00 ml of vinegar (active ingredient is acetic acid) is titrated to the endpoint using 19.32 ml of 0.250 M sodium hydroxide. What is the molarity of acetic acid in the vinegar? YOU MUST SHOW YOUR WORK. NOTE: MA x VA = MB x VBarrow_forward424 Repon Sheet Rates of Chemical Reactions : Rate and Order of 1,0, Deception B. Effect of Temperature BATH TEMPERATURE 35'c Yol of Oh نام Time 485 Buret rend ing(n) 12 194 16. 6 18 20 10 22 24 14 115 95 14738 2158235 8:26 CMS 40148 Total volume of 0, collected Barometric pressure 770-572 ml mm Hg Vapor pressure of water at bath temperature (see Appendix L) 42.2 Slope Compared with the rate found for solution 1, there is Using the ideal gas law, calculate the moles of O; collected (show calculations) times faster 10 Based on the moles of O, evolved, calculate the molar concentration of the original 3% 1,0, solution (sho calculations)arrow_forwardSteps and explanation pleasearrow_forward
- Use diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward15) Create Lewis structure Br Brarrow_forward
- LIOT S How would you make 200. mL of a 0.5 M solution of CuSO4 5H2O from solid copper (II) sulfate? View Rubricarrow_forwardSteps and explantions pleasearrow_forwardMatch the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





