
Interpretation:
The molecular shapes and hybrid orbitals of
Concept introduction:
The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). The hybridization of an atom indicates the molecular geometry of a molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
Answer to Problem 66SSC
In
Explanation of Solution
In the
Number of valence electron = Number of atom P (valence electron in P) + Number of atom F (valence electron in F) =
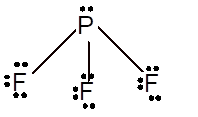
With sp3 hybridization, the geometry must be tetrahedral but the presence of one lone pair on the central P atom alters the standard geometry of the molecule to pyramid shape due to repulsion between the lone pair and bond pair of the molecule.
In the
Number of valence electron = Number of atom P (valence electron in P) + Number of atom F (valence electron in F) =
Hence the Lewis structure must be:
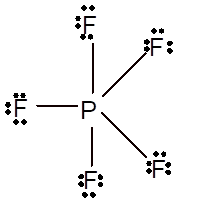
Thus, in
The change in hybridization alters the molecular geometry of the molecule.
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Applications and Investigations in Earth Science (9th Edition)
Cosmic Perspective Fundamentals
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: A Molecular Approach (4th Edition)
Microbiology: An Introduction
Chemistry: The Central Science (14th Edition)
- 2. Calculate the branching ratio of the reaction of the methyl peroxy radical with either HO, NO 298K) (note: rate constant can be found in the tropospheric chemistry ppt CH,O,+NO-HCHO+HO, + NO, CH₂O+HO, CH₂00H +0₂ when the concentration of hydroperoxyl radical is DH01-1.5 x 10 molecules and the nitrogen oxide maxing ratio of 10 ppb when the concentration of hydroperoxyl radicalis [H0] +1.5x10 molecules cm" and the nitrogen oxide mixing ratio of 30 p Under which condition do you expect more formaldehyde to be produced and whyarrow_forwardIndicate the product of the reaction of benzene with 1-chloro-2,2-dimethylpropane in the presence of AlCl3.arrow_forwardIn what position will N-(4-methylphenyl)acetamide be nitrated and what will the compound be called.arrow_forward
- DATA: Standard Concentration (caffeine) mg/L Absorbance Reading 10 0.322 20 0.697 40 1.535 60 2.520 80 3.100arrow_forwardIn what position will p-Toluidine be nitrated and what will the compound be called.arrow_forwardIn what position will 4-methylbenzonitrile be nitrated and what will the compound be called.arrow_forward
- In what position will benzenesulfonic acid be nitrated?arrow_forwardIf compound A reacts with an excess of methyl iodide and then heated with aqueous Ag₂O, indicate only the major products obtained. Draw their formulas. A Harrow_forwardExplanation Check 1:01AM Done 110 Functional Groups Identifying and drawing hemiacetals and acetals In the drawing area below, create a hemiacetal with 1 ethoxy group, 1 propoxy group, and a total of 9 carbon atoms. Click and drag to start drawing a structure. ✓ $ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Sarrow_forward
- Write the systematic name of each organic molecule: CI structure CI CI Explanation CI ठ CI Check B ☐ 188 F1 80 name F2 F3 F4 F5 F6 60 F7 2arrow_forwardWrite the systematic name of each organic molecule: structure i HO OH Explanation Check name ☐ ☐arrow_forwardX 5 Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. CI Br Br Br 0 None of these molecules have a total of five ẞ hydrogens. Explanation Check esc F1 F2 tab caps lock fn Q @2 A W # 3 OH O OH HO © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility IK F7 F7 F8 TA F9 F10 & 6 28 * ( > 7 8 9 0 80 F3 O F4 KKO F5 F6 S 64 $ D % 25 R T Y U பட F G H O J K L Z X C V B N M H control option command P H F11 F12 + || { [ command optionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





