
Concept explainers
Interpretation:
From the following carbon-nitrogen bonds, shorter and strongest bond needs to be identified.
 and
and 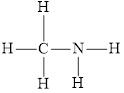
Concept introduction:
The strength of a covalent bond depends on the distance between the bonded nuclei. Bond length is the distance between the maximum attractions of
Answer to Problem 86A
Triple bond in is the strongest and shortest bond than C-N bond of
is the strongest and shortest bond than C-N bond of 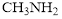
Explanation of Solution
Bond strength and bond length are related to each other. If bond length is shorter, then the bond is stronger.  contain one triple bind whereas
contain one triple bind whereas 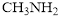 contains 6 triple bonds. With increase in the number of shared electron pair, the bond length decreases. In triple bond, two atoms share three electrons pair. Hence, triple bond of
contains 6 triple bonds. With increase in the number of shared electron pair, the bond length decreases. In triple bond, two atoms share three electrons pair. Hence, triple bond of  is the strongest and shortest bond.
is the strongest and shortest bond.
In double bond, two atoms share two electrons pair while in single bond one electrons pair is shared.
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Biology: Life on Earth (11th Edition)
Campbell Biology (11th Edition)
Human Anatomy & Physiology (2nd Edition)
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





