
Concept explainers
Interpretation:
The reason for the
Concept introduction:
The asymmetric structure and presence of polar covalent bonds confirm that the molecule is polar in nature. The molecule having some dipole moment is termed as a polar molecule and the molecule with no dipole moment or zero dipole moment is identified as a non-polar molecule.
Answer to Problem 121A
The charge distribution at any distance from its center is equal but opposite in direction. Therefore,
Explanation of Solution
Let’s assume a bond is formed between two elements A and B. For a bond to be ionic, the electro negativity difference
In
The electro negativity difference
The given molecule
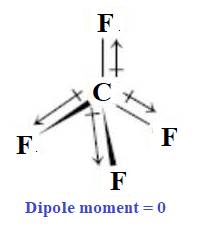
In
The charge distribution at any distance from its center is equal but opposite in direction. Therefore,
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
The Cosmic Perspective (8th Edition)
Biology: Life on Earth (11th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Human Anatomy & Physiology (2nd Edition)
Chemistry: Structure and Properties (2nd Edition)
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
- I need the nomenclature of this compound.arrow_forwardI need the nomenclature of this compoundarrow_forward2. Name the following hydrocarbons. (9 marks) a) HHHHHHHH H-C-C- H-O-S b) HCEC-CH3 H H H H H d) c) H C=C- H H H e) CH3 CH3 CH2CH=CH-CH=CHCH3 HHHH H-C-C-C-C-H H HH H f) large CH2CH3 pola H3C section lovels tower, able ocart firs g) Tower H3C-CH2 then in H3C-CH-CH-CH3 enblbano bne noitsidab Copyright © 2008. Durham Continuing Education CH3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





