
Concept explainers
Interpretation:
The molecular formula for luminol is to be written. The Lewis structure of luminol is to be drawn.
Concept introduction:
In the molecular formula, the element symbols and the number of the subscript tells the type and the number of each atom in a molecule.
There are so many methods to represent a molecule. In the ball-and-stick method, the atoms of each element are depicted by a ball and the bond is depicted by a stick. In the structural formula method, the atoms of each element are depicted by letter symbols and bond is used to show relative position.
Answer to Problem 143A
The molecular formula for luminol is
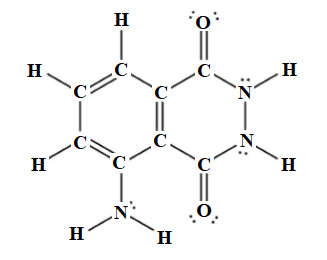
Explanation of Solution
The ball and stick model of luminol is shown below.
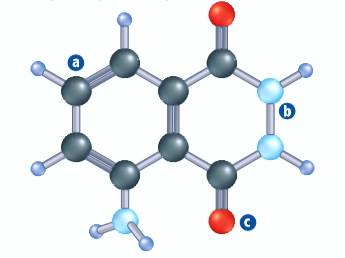
In the above ball and stick model, the black balls represent carbon atoms, red balls represent oxygen atoms, light blue balls represent nitrogen atoms, and dark blue balls represent hydrogen atoms. The molecular structure of luminol is shown below.
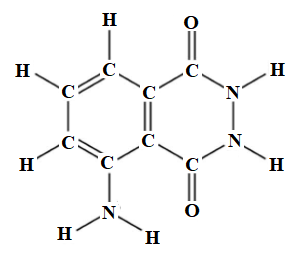
There are eight carbon atoms, seven hydrogen atoms, three nitrogen atoms, and two oxygen atoms. Therefore, the molecular formula for luminol is
The number of valence electrons in luminol is the sum of the valence electron in carbon, hydrogen, nitrogen, and oxygen atoms. The valence electron in carbon is four, the valence electron in hydrogen is one, the valence electron in nitrogen is five and the valence electron in oxygen is six. Therefore, the valence electron in luminol is calculated as shown below.
The number of electron pair is calculated as shown below.
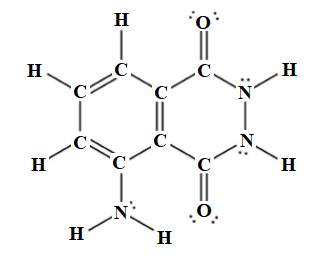
Among thirty-three electron pairs, twenty-six electron pairs are involved in bonding and seven electron pairs remain as lone pairs. The Lewis structure of luminol is drawn below.
The Lewis structure of luminol is drawn below.
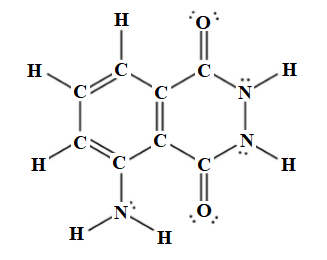
The molecular formula for luminol is
. The Lewis structure of luminol is drawn as follows:
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
Chemistry: Structure and Properties (2nd Edition)
Human Anatomy & Physiology (2nd Edition)
Anatomy & Physiology (6th Edition)
Microbiology: An Introduction
- Name the molecules & Identify any chiral center CH3CH2CH2CHCH₂CH₂CH₂CH₂ OH CH₂CHCH2CH3 Br CH3 CH3CHCH2CHCH2CH3 CH3arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forward
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





