
Concept explainers
(a)
Interpretation:
Molecular shape and bond angle of A-B needs to be explained.
Concept introduction:
A molecule’s physical and chemical properties are determined through its shape. The shapes of the reactant molecules play an important role in determining whether they come close to react or not. The densities of electrons generated by the overlap of the mutual electrons' orbitals define the molecular structure.
(a)
Answer to Problem 108A
The shape of the molecule A-B is linear and its bond angle is
Explanation of Solution
The A-B molecule contains one bonding pair. Hence, its molecular shape is linear and its bond angle is
(b)
Interpretation:
Molecular shape and bond angle of A-B-A needs to be explained.
Concept introduction:
A molecules physical and chemical properties are determines through its shape. The shapes of the reactant molecules play an important role in determining whether they come close to react or not. The densities of electrons generated by the overlap of the mutual electrons' orbitals define the molecular structure.
(b)
Answer to Problem 108A
The shape of the molecule A-B-B is linear and its bond angle is
Explanation of Solution
The A-B-A molecule contains two bonding pair. Hence, its molecular shape is linear and its bond angle is
(c)
Interpretation:
Molecular shape and bond angle of following molecules needs to be explained.
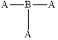
Concept introduction:
A molecules physical and chemical properties are determines through its shape. The shapes of the reactant molecules play an important role in determining whether they come close to react or not. The densities of electrons generated by the overlap of the mutual electrons' orbitals define the molecular structure.
(c)
Answer to Problem 108A
The shape of the molecule is trigonal planar and its bond angle is
Explanation of Solution
The molecule contains three pair of bonding electrons. Hence, its molecular shape is trigonal planar and its bond angle is
(d)
Interpretation:
Molecular shape and bond angle of following molecule needs to be explained.
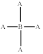
Concept introduction:
A molecules physical and chemical properties are determines through its shape. The shapes of the reactant molecules play an important role in determining whether they come close to react or not. The densities of electrons generated by the overlap of the mutual electrons' orbitals define the molecular structure.
(d)
Answer to Problem 108A
The shape of the molecule is tetrahedral and its bond angle is
Explanation of Solution
The molecule contains four bonding pairs. Hence, its molecular shape is tetrahedral and its bond angle is
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Biology in Focus (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
Organic Chemistry (8th Edition)
Microbiology: An Introduction
Anatomy & Physiology (6th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Curved arrows are used to illustrate the flow of electrons. Using the provided structures, draw the curved arrows that epict the mechanistic steps for the proton transfer between a hydronium ion and a pi bond. Draw any missing organic structures in the empty boxes. Be sure to account for all lone-pairs and charges as well as bond-breaking and bond-making steps. 2 56°F Mostly cloudy F1 Drawing Arrows > Q Search F2 F3 F4 ▷11 H. H : CI: H + Undo Reset Done DELLarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbons. Draw out the benzene ring structure when doing itarrow_forward1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series. 2) Calculate the ionization energy of He* and L2+ ions in their ground states. 3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.arrow_forward
- Calculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forward
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





