
Concept explainers
Interpretation:
The compound that does not have a bent shape is to be identified.
Concept introduction:
The molecular shape of a molecule is determined via a model referred as valence shell electron pair repulsion (VSEPR) model. According to this model, in a molecule, the atoms and the lone pairs around the central atom are arranged in such a manner that minimizes the repulsion.
Answer to Problem 9STP
The compound that does not have a bent shape is
Explanation of Solution
Reason for correct option: The
The bonding in
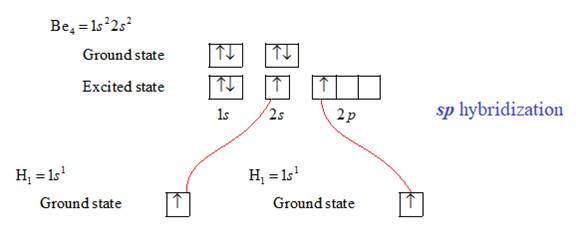
Therefore, each hydrogen atom is attached with beryllium atom via single bond. There is no lone pair of electrons. Therefore, the shape of
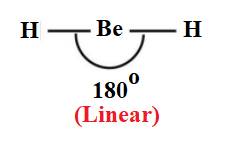
Therefore, correct option is option (A).
Reason for incorrect option: The compound
The compound
The compound
Chapter 8 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Cosmic Perspective Fundamentals
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Anatomy & Physiology (2nd Edition)
Microbiology with Diseases by Body System (5th Edition)
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
- I need the nomenclature of this compound.arrow_forwardI need the nomenclature of this compoundarrow_forward2. Name the following hydrocarbons. (9 marks) a) HHHHHHHH H-C-C- H-O-S b) HCEC-CH3 H H H H H d) c) H C=C- H H H e) CH3 CH3 CH2CH=CH-CH=CHCH3 HHHH H-C-C-C-C-H H HH H f) large CH2CH3 pola H3C section lovels tower, able ocart firs g) Tower H3C-CH2 then in H3C-CH-CH-CH3 enblbano bne noitsidab Copyright © 2008. Durham Continuing Education CH3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





