
Concept explainers
Interpretation:
The difference of
Concept introduction:
The hydrocarbon compounds contain compound that are made up of only hydrogen and carbon atoms.
Explanation of Solution
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon whereas hydrocarbon compounds that contains multiple bond(s) between carbon atom(s) are said to be
The general formula of:
Alkane is
Alkene is
Alkyne is
The number of H atoms in alkene is 2 units less than that in alkane and the number of H atoms in alkyne is 2 units less than that in alkene and 4 units less than that in alkane.
For example:
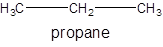
The molecular formula of propane is
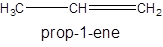
The molecular formula of propene is
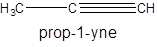
The molecular formula of propyne is
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Biology in Focus (2nd Edition)
Anatomy & Physiology (6th Edition)
Microbiology: An Introduction
Introductory Chemistry (6th Edition)
- 5. A buffer consists of 0.45 M NH, and 0.25 M NH-CI (PK of NH 474) Calculate the pH of the butter. Ans: 9.52 BAS PH-9.26 +10g (10.95)) 14-4.59 PH=4.52 6. To 500 ml of the buffer on #5 a 0.20 g of sample of NaOH was added a Write the net ionic equation for the reaction which occurs b. Should the pH of the solution increase or decrease sightly? Calculate the pH of the buffer after the addition Ans: 9.54arrow_forwardExplain the inductive effect (+I and -I) in benzene derivatives.arrow_forwardThe inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?arrow_forward
- 19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward7. Helparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





