
Concept explainers
Interpretation:
The effect of intermolecular attractions on the boiling and freezing point of the substance (water and methane) needs to be explained.
Concept introduction:
The strength of intermolecular forces present between the molecules influences the properties of the substance like boiling point, freezing point, etc. If the strength of the intermolecular forces is strong, then it results in higher boiling point and low vapor pressure of the liquid.
Explanation of Solution
The structure of water,
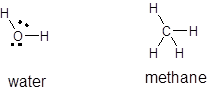
Methane is a non-polar molecule whereas water is a polar molecule. The force of attraction between polar molecules are greater than those of non-polar molecule so, methane being non-polar will possess less attraction between methane molecules but water molecules possess polar
Due to presence of an oxygen atom in water, the formation of intermolecular hydrogen bonding takes place in which O atom of one molecule of water is bonded to H atom of another molecule of water. The hydrogen bonding in
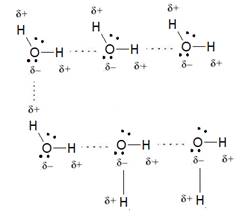
Hence, the water molecule has higher boiling and freezing point than methane.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Essential Biology (7th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology with Diseases by Body System (5th Edition)
Cosmic Perspective Fundamentals
Organic Chemistry (8th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- Steps and explanation please. Add how to solve or target similar problems.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardThis organic molecule is dissolved in an acidic aqueous solution: OH OH A short time later sensitive infrared spectroscopy reveals the presence of a new C = O stretch absorption. That is, there must now be a new molecule present with at least one C = O bond. In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. Videos 849 Explanation Check C Click and drag to start dwing a structure. # 3 MAR 23 Add/Remove steparrow_forward||| 7:47 ull 57% ← Problem 19 of 48 Submit Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this carbocation rearrangement. Include all lone pairs and charges as appropriate. H 1,2-alkyl shift +arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardBelow is the SN1 reaction of (S)-3-chlorocyclohexene and hydroxide (OH). Draw the missing curved arrows, lone pairs of electrons, and nonzero formal charges. In the third box, draw the two enantiomeric products that will be produced. 5th attempt Please draw all four bonds at chiral centers. Draw the two enantiomeric products that will be produced. Draw in any hydrogen at chiral centers. 1000 4th attempt Feedback Please draw all four bonds at chiral centers. 8. R5 HO: See Periodic Table See Hint H Cl Br Jid See Periodic Table See Hintarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





