
Interpretation:
Saturated and
Concept introduction:
Compounds that contains only carbon and hydrogen atoms are said to be hydrocarbons. The hydrocarbons can be saturated or unsaturated classified on the basis of the type of bond present in the compound.
Explanation of Solution
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon whereashydrocarbon compounds that containsmultiple bond(s) between carbon atom(s) are said to be unsaturated hydrocarbon. Compounds containing single bonds are said to be
For example:
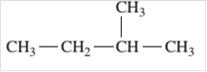
Since, the compound contains only single bonds so, it is an alkane (saturated hydrocarbon).
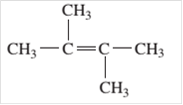
Since, the compound contains double bond between two carbon atoms so, it is an alkene (unsaturated hydrocarbon).
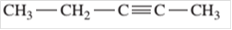
Since, the compound contains triple bond between two carbon atoms so, it is an alkyne (unsaturated hydrocarbon).
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Biological Science (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
Microbiology: An Introduction
Campbell Essential Biology (7th Edition)
Biology: Life on Earth (11th Edition)
The Cosmic Perspective (8th Edition)
- How many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





