
Interpretation:
The structure, history of development and use of tetraethyllead needs to be discussed and the reason for discontinuation of its use in United States needs to be explained. Also, whether it is still in use elsewhere in the world as a gasoline additive should be determined.
Concept introduction:
Hydrocarbons make good wide variety of fuels and consumer goods as their covalent bonds store large amount of energy, on burning the molecule a large amount of energy is released.
Explanation of Solution
Tetraethyllead is an
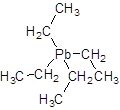
It was first found in 1920s, synthesized by the action of ethyl chloride on a powdered alloy of lead.
This compound was used in gasoline as a chief antiknock agent in car engines.
In engines, on heating the bonds between ethyl groups and lead breaks down and forms lead atom which on combustion forms lead oxide (PbO). The use of tetraethyllead was discontinued once it was found to be poisoning due to toxic metal lead in the compound.
Yes, it is still in use elsewhere in the world as a gasoline additive as it is used in gasolines for planes.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
Biology: Life on Earth (11th Edition)
Campbell Biology (11th Edition)
Anatomy & Physiology (6th Edition)
- Can someone help me whats the issue?arrow_forwarda. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression: AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T A. Calculate the value of AS for the process. B. Next, use the Gibbs-Helmholtz equation: (a(AG/T)) ΔΗ - T2 to calculate the value of AH for the process.arrow_forwardNonearrow_forward
- ASP please....arrow_forwardNonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forward
- Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





