
Interpretation:
The IUPAC name of the following compound should be determined:
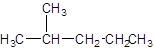
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be
Answer to Problem 13SSC
2-methylpentane.
Explanation of Solution
In order to give the IUPAC name to the alkane following steps are followed:
1. The parent (longest) continuous carbon chain is identified.
2. The ending of the parent chain for alkane (-e) is -ane.
3. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:

The given structure is:
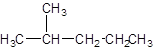
The parent chain contains 1 substituent so numbering is done in such a way that substituent gets lower number as shown:
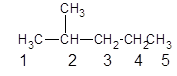
Since, the parent chain contains 5 carbon atoms and only single bonds are present so, the parent chain is pentane. Since, the substituent,
Interpretation:
The IUPAC name of the following compound should be determined:
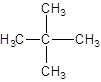
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 13SSC
2, 2-dimethylpropane.
Explanation of Solution
The given structure is:
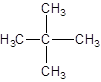
The parent chain contains 2 substituents so numbering is done in such a way that substituents gets lower number as shown:
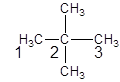
Since, the parent chain contains 3 carbon atoms and only single bonds are present so, the parent chain is propane. Since, the substituents,
Interpretation:
The IUPAC name of the following compound should be determined:
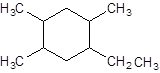
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkanes.
Answer to Problem 13SSC
1-ethyl-2, 4, 5-trimethylcyclohexane.
Explanation of Solution
In order to give the IUPAC name to the cycloalkane following steps are followed:
1. The parent (longest) continuous carbon chain is identified.
2. The prefix -cyclo is used before the name of alkane for a ring.
3. If the attached alkyl chain possesses greater number of carbon atoms, then alkyl chain is considered as primary parent chain.
4. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
5. Hyphen is used to connect the number to the name.
The given structure is:
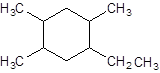
The parent chain contains a ring of six carbon atoms and 4 substituents so numbering is done in such a way that substituents gets lower number as shown:
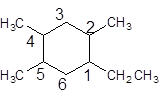
Since, the parent chain contains a ring of six carbon atoms and only single bonds are present so, the parent chain is cyclohexane. Since, the substituents, one
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Campbell Biology: Concepts & Connections (9th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Essential Biology (7th Edition)
Applications and Investigations in Earth Science (9th Edition)
Cosmic Perspective Fundamentals
- Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- Concentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forwardExplain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forward
- Draw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forwardDraw the condensed structural formula and line-angle formula for each: 2,3-dimethylheptane 3-bromo-2-pentanol 3-isopropyl-2-hexene 4-chlorobutanoic acidarrow_forward
- Record the IUPAC names for each of the structures shown below. a) b) c) OH d) OH e)arrow_forwardA solution of 14 g of a nonvolatile, nonelectrolyte compound in 0.10 kg of benzene boils at 81.7°C. If the BP of pure benzene is 80.2°C and the K, of benzene is 2.53°C/m, calculate the molar mass of the unknown compound. AT₁ = Km (14)arrow_forwardPlease help me answer the following questions. My answers weren't good enough. Need to know whyy the following chemicals were not used in this experiment related to the melting points and kf values. For lab notebook not a graded assignments.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





