
Concept explainers
How does the structure of a cycloalkane differ from that of a straight-chain or branched-chain
Interpretation:
The difference in structure of cycloalkane from that of a straight chain or branched-chain alkane needs to be explained.
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkanes.
Answer to Problem 53A
The difference in structure of cycloalkane from that of a straight chain or branched-chain alkane is that cycloalkanes contains 2 H atoms less than that of straight chain or branched-chain alkane.
Explanation of Solution
The general formula of alkane is
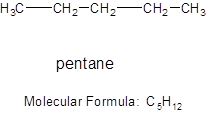
The branched chain of 5 carbon atoms will be:
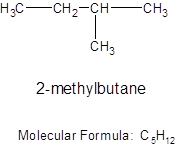
For a cycloalkane formed from 5 carbon atoms, the structure and the molecular formula will be:
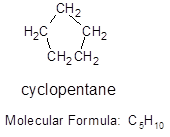
Cyclopentane that means it contains 5 carbon atoms and 10 hydrogen atoms.
Since, in cycloalkane the number of hydrogen atoms are less from alkane by 2 units so, the general formula of cycloalkane will be:
Hence, the difference in structure of cycloalkane from that of a straight chain or branched-chain alkane is that cycloalkanes contains 2 H atoms less than that of straight chain or branched-chain alkane.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Genetic Analysis: An Integrated Approach (3rd Edition)
Human Anatomy & Physiology (2nd Edition)
Cosmic Perspective Fundamentals
Campbell Biology: Concepts & Connections (9th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Organic Chemistry (8th Edition)
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forwardExplain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forward
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





