
Concept explainers
a.
Interpretation:
The name of the following structural formula needs to be determined:
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be
a.
Answer to Problem 55A
Pentane.
Explanation of Solution
In order to give the name to the alkane following steps are followed:
1. The parent (longest) continuous carbon chain is identified.
2. The ending of the parent chain for alkane (-e) is -ane.
3. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The given structure is:
The parent chain contains 5 carbon as shown:
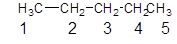
Since, the parent chain contains 5 carbon atoms and only single bonds are present so, the name of the compound is pentane.
b.
Interpretation:
The name of the following compound should be determined:
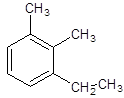
Concept introduction:
The ring structures of the compound having uncommon stability due to delocalized pi electron density shared in between all the carbon atoms of the ring is said to be an aromatic compound.
b.
Answer to Problem 55A
1-ethyl-2, 3-dimethylbenzene.
Explanation of Solution
In order to give the name to the multiple substituted
- For single substituted aromatic compound (when the substituent contains six or fewer carbons), the name of the substituted group is written first followed by the name of the aromatic compound.
- For single substituted aromatic compound (when the substituent contains more than six carbons), the name of the aromatic compound is written first followed by the name of the substituted group.
- For single substituted aromatic compound, the numbering on the ring is done in such a way that the multiple substituents get the lowest number.
The given structure is:
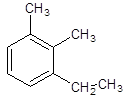
The given structure is trisubstituted substituted aromatic compound, benzene that contains two carbon chain, ethyl and 2 substituents as methyl so, numbering is done in a way:
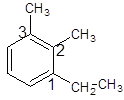
Hence, the name of the compound is 1-ethyl-2, 3-dimethylbenzene.
c.
Interpretation:
The name of the following structural formula needs to be determined:
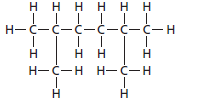
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
c.
Answer to Problem 55A
2, 5-dimethylhexane.
Explanation of Solution
The given structure is:
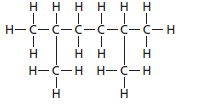
The parent chain contains 6 carbon atoms and 4 substituents so, the numbering is done as shown:
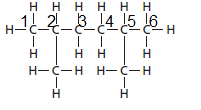
Since, the parent chain contains 6 carbon atoms and 2 methyl substituents at position 2 and 5 so, the name of the compound is 2, 5-dimethylhexane.
d.
Interpretation:
The name of the following structural formula needs to be determined:
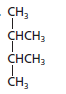
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
d.
Answer to Problem 55A
2, 3-dimethylbutane.
Explanation of Solution
The given structure is:
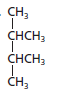
The parent chain contains 4 carbon atoms and 2 substituents so, the numbering is done as shown:
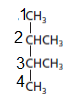
Since, the parent chain contains 4 carbon atoms and 2 methyl substituents at position 2 and 3 so, the name of the compound is 2, 3-dimethylbutane.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
College Physics: A Strategic Approach (3rd Edition)
The Cosmic Perspective (8th Edition)
Anatomy & Physiology (6th Edition)
Campbell Biology (11th Edition)
Introductory Chemistry (6th Edition)
- LTS Solid: AT=Te-Ti Trial 1 Trial 2 Trial 3 Average ΔΗ Mass water, g 24.096 23.976 23.975 Moles of solid, mol 0.01763 001767 0101781 Temp. change, °C 2.9°C 11700 2.0°C Heat of reaction, J -292.37J -170.473 -193.26J AH, kJ/mole 16.58K 9.647 kJ 10.85 kr 16.58K59.64701 KJ mol 12.35k Minimum AS, J/mol K 41.582 mol-k Remember: q = mCsAT (m = mass of water, Cs=4.184J/g°C) & qsin =-qrxn & Show your calculations for: AH in J and then in kJ/mole for Trial 1: qa (24.0969)(4.1845/g) (-2.9°C)=-292.37J qsin = qrxn = 292.35 292.37J AH in J = 292.375 0.2923kJ 0.01763m01 =1.65×107 AH in kJ/mol = = 16.58K 0.01763mol mol qrx Minimum AS in J/mol K (Hint: use the average initial temperature of the three trials, con Kelvin.) AS=AHIT (1.65×10(9.64×103) + (1.0 Jimaiarrow_forwardFor the compound: C8H17NO2 Use the following information to come up with a plausible structure: 8 This compound has "carboxylic acid amide" and ether functional groups. The peaks at 1.2ppm are two signals that are overlapping one another. One of the two signals is a doublet that represents 6 hydrogens; the other signal is a quartet that represents 3 hydrogens.arrow_forwardVnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling point, choose 2 next to the substance with the next highest boiling point, and so on. substance C D chemical symbol, chemical formula or Lewis structure. CH,-N-CH, CH, H H 10: H C-C-H H H H Cale H 10: H-C-C-N-CH, Bri CH, boiling point (C) Сен (C) B (Choosearrow_forward
- Please help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!arrow_forwardQ1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br "CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardExperiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward
- (15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forwardQ7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





