
Concept explainers
Interpretation:
The condensed structural formula of 1, 4-diethylcyclohexene needs to be drawn.
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkenes (containing one or more double bond in the ring).
Answer to Problem 62A
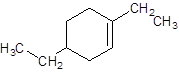
Explanation of Solution
The given name is 1, 4-diethylcyclohexene that means the parent chain contains cyclic ring of 6 carbon atoms with 1 double bond in the ring and -diethyl represents the presence of two ethyl substituents at position 1 and 4. So, the structure of 1, 4-diethylcyclohexene is:
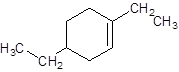
Interpretation:
The condensed structural formula of 2, 4-dimethyl-1-octene needs to be drawn.
Concept introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be
Answer to Problem 62A
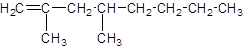
Explanation of Solution
The given name of the compound is 2, 4-dimethyl-1-octene, the oct in the name represents the parent chain of 8 carbon atoms and ene represents the presence of double bond at position 1 and -dimethyl represents the presence of 2 methyl substituents at position 2 and 4. So, the structure of 2, 4-dimethyl-1-octene is:
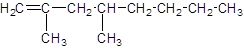
Interpretation:
The condensed structural formula of 2, 2-dimethyl-3-hexyne needs to be drawn.
Concept introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
Answer to Problem 62A
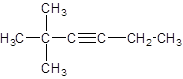
Explanation of Solution
The given name of the compound is 2, 2-dimethyl-3-hexyne, the hex in the name represents the parent chain of 6 carbon atoms and yne represents the presence of triple bond at position 3 and -dimethyl represents the presence of 2 methyl substituents at position 2. So, the structure of 2, 2-dimethyl-3-hexyne is:
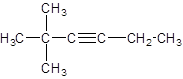
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Chemistry: Structure and Properties (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
Human Anatomy & Physiology (2nd Edition)
Biology: Life on Earth (11th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- Indicate the names of these compounds (if they exist). 0: HỌC—NH CH3CH2-CH2arrow_forwardN Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forwardThe conjugate base of alkanes is called alkides. Correct?.arrow_forward
- Name these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forwardHH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forward
- Name these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forwardClassify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





