
Concept explainers
Interpretation:
The name of compound should be determined for the following skeletal structure.
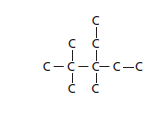
- 2, 2, 3-trimethyl-3-ethylpentane
- 3-ethyl-3, 4, 4-trimethylpentane
- 2-butyl-2-ethylbutane
- is 3-ethyl-2, 2, 3-trimethylpentane
- 2, 2-dimethyl, 3-diethyl, 3-methylpropane
Concept introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming
- First choose the longest continuous chain of carbon atoms known as parent chain and determines the base name of alkane.
- The numbering of parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
Answer to Problem 15STP
Option (D) is correct that is 3-ethyl-2, 2, 3-trimethylpentane.
Explanation of Solution
The skeletal structure of compound:
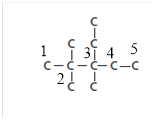
- The longest continuous chain contains five carbon atoms and the base name is pentane.
- Two substituents that are two methyl groups are present on second carbon atom of the chain.
- Two substituents that are ethyl and methyl group are present on third carbon atom of the chain.
- The numbering for the substituents is given as: 2, 2, 3-trimethyl and 3-ethyl
- Since, ethyl is starting with alphabet “e” thus, comes first in the naming of the compound.
Therefore, the name of the compound is 3-ethyl-2, 2, 3-trimethylpentane.
Hence, option (D) is correct.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Biology (11th Edition)
Cosmic Perspective Fundamentals
Campbell Biology: Concepts & Connections (9th Edition)
Microbiology: An Introduction
Chemistry: Structure and Properties (2nd Edition)
Human Physiology: An Integrated Approach (8th Edition)
- For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardGiven the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- k₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forwardk₁ Given the reaction R₂ R + R, indicate k-1 (A). the rate law with respect to R2: (B). the rate law with respect to R: d[R₂] dt d[R] dtarrow_forwardGiven the reaction R+ Q → P, indicate (A). the rate law with respect to P: (B). the rate law with respect to R: (C). the rate law with respect to Q: d[P] dt d[R] dt d[Q] dtarrow_forward
- The reaction for obtaining NO2 from NO and O2 has the rate equation: v = k[NO]2[O2]. Indicate which of the following options is correct.(A). This rate equation is inconsistent with the reaction consisting of a single trimolecular step.(B). Since the overall order is 3, the reaction must necessarily have some trimolecular step in its mechanism.(C). A two-step mechanism: 1) NO + NO ⇄ N2O2 (fast); 2) N2O2 + O2 → NO2 + NO2 (slow).(D). The mechanism must necessarily consist of three unimolecular elementary steps with very similar rate constants.arrow_forwarda. What is the eluent used in the column chromatography here (a “silica plug filtration” is essentially a very short column)? b. The spectroscopy of compound 5b is described in the second half of this excerpt, including 1H-NMR and 13C-NMR (which you will learn about in CHEM 2412L), MS (which you will learn about later in CHEM 2411L) and IR. One of the IR signals is at 3530 cm-1. What functional group does this indicate might be present in compound 5b?arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- a. The first three lines of this procedure describe the reaction used to make compound 5b. In the fourth line, hexane and sodium bicarbonate are added. What organic lab technique is being used here? b. What is the purpose of the Na2SO4? c. What equipment would you use to “concentrate [a solution] under reduced pressure”?arrow_forwardWhen N,N-dimethylaniline is treated with bromine both the ortho and para products are observed. However when treated with a mixture of nitric acid and sulfuric acid only the meta product is observed. Explain these results and support your answer with the appropriate drawings *Hint amines are bases* N HNO3 H2SO4 N NO2 N Br2 N Br + N 8-8-8 FeBr3 Brarrow_forwardDraw a mechanism that explains the formation of compound OMe SO3H 1. Fuming H2SO4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





