
Concept explainers
(a)
Interpretation:
The name of the following compound should be determined:
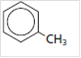
Concept introduction:
The ring structures of the compound having uncommon stability due to delocalized pi electron density shared in between all the carbon atoms of the ring is said to be an
(a)
Answer to Problem 77A
Methylbenzene.
Explanation of Solution
The main group in the given compound is benzene which has a methyl group substituent. The name of the compound should be written in such a way that name of substituent is written first followed by the name of the main group. So, the name of the compound is methylbenzene, which is also known as toluene.
(b)
Interpretation:
The name of the following compound should be determined:
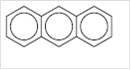
Concept introduction:
The ring structures of the compound having uncommon stability due to delocalized pi electron density shared in between all the carbon atoms of the ring is said to be an aromatic compound.
(b)
Answer to Problem 77A
Anthracene.
Explanation of Solution
The given compound is a fused benzene ring compound, in “fused” benzene rings molecule, two or more benzene rings have two carbon atoms in common. The semi-trivial name of the given compound is anthracene.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
The Cosmic Perspective (8th Edition)
Campbell Biology in Focus (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Campbell Biology (11th Edition)
Anatomy & Physiology (6th Edition)
- The heat of combustion for ethane, C2H6C2H6 , is 47.8 kJ/g. How much heat is produced if 1.65 moles of ethane undergo complete combustion?arrow_forwardReview of this week's reaction: H2NCN (cyanamide) + CH3NHCH2COOH (sarcosine) + NaCl, NH4OH, H2O ----> H2NC(=NH)N(CH3)CH2COOH (creatine) Q7. Draw by hand the reaction of creatine synthesis listed above using line structures without showing the Cs and some of the Hs, but include the lone pairs of electrons wherever they apply. (4 pts) Q8. Considering the Zwitterion form of an amino acid, draw the Zwitterion form of Creatine. (2 pts) Q9. Explain with drawing why the C—N bond shown in creatine structure below can or cannot rotate. (3 pts)arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Please help me answer a. Please and thank you I advance.arrow_forwardDraw both of the chair flips for both the cis and trans isomers for the following compounds: 1,4-diethylcyclohexane 1-methyl-3-secbutylcyclohexanearrow_forwardPpplllleeeaaasssseeee hellppp wiithhh thisss physical chemistryyyyy I talked like this because AI is very annoyingarrow_forward
- For this question, if the product is racemic, input both enantiomers in the same Marvin editor. A) Input the number that corresponds to the reagent which when added to (E)-but-2-ene will result in a racemic product. Input 1 for Cl, in the cold and dark Input 2 for Oy followed by H₂O, Zn Input 3 for D₂ with metal catalyst Input 4 for H₂ with metal catalyst B) Draw the skeletal structure of the major organic product made from the reagent in part A Marvin JS Help Edit drawing C) Draw the skeletal structure of the major organic product formed when (2)-but-2-ene is treated with peroxyacetic acid. Marvin 35 Helparrow_forwardMichael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forwardRank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





