
(a)
Interpretation:
The subshell model of Na, sodium element, needs to be drawn.
Concept introduction:
A subshell is defined as one or more orbitals in a shell with same energy level. They have different shapes and shape of an orbital is identified by magnetic quantum number.
Answer to Problem 6E
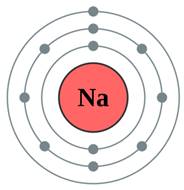
Explanation of Solution
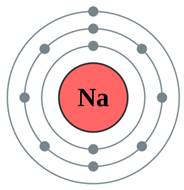
The
(b)
Interpretation:
The subshell model of Ne, 10 element, needs to be drawn.
Concept introduction:
A subshell is defined as one or more orbitals in a shell with same energy level. They have different shapes and shape of an orbital is identified by magnetic quantum number.
Answer to Problem 6E
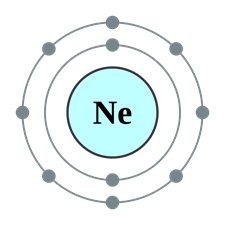
Explanation of Solution
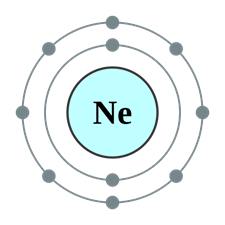
The atomic number of Neon is 10 and its electronic configuration is
(c)
Interpretation:
The subshell model of Carbon, C element, needs to be drawn.
Concept introduction:
A subshell is defined as one or more orbitals in a shell with same energy level. They have different shapes and shape of an orbital is identified by magnetic quantum number.
Answer to Problem 6E
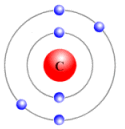
Explanation of Solution
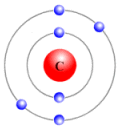
The atomic number of Carbon is 6 and its electronic configuration is
(d)
Interpretation:
The subshell model of Vanadium, V element, needs to be drawn.
Concept introduction:
A subshell is defined as one or more orbitals in a shell with same energy level. They have different shapes and shape of an orbital is identified by magnetic quantum number.
Answer to Problem 6E
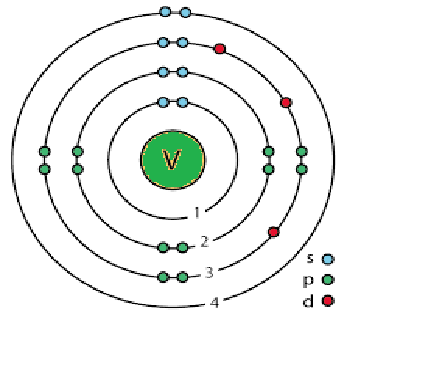
Explanation of Solution
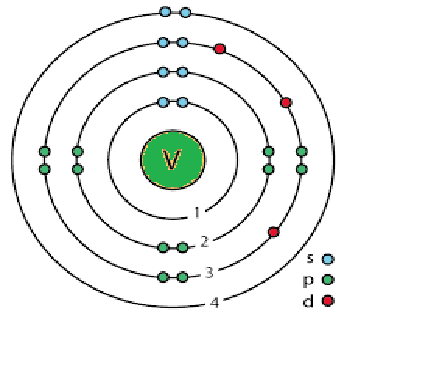
The atomic number of Vanadium is 23 and its electronic configuration is
. Above diagram shows the arrangement of electrons in the different subshells.
Chapter U1 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Microbiology with Diseases by Body System (5th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Applications and Investigations in Earth Science (9th Edition)
Anatomy & Physiology (6th Edition)
- What is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forwardΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forward
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





