
Concept explainers
(a)
Interpretation:
Whether NAD+ is involved in (1) glycerol
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
Nicotinamide adenine dinucleotide is associated with the
(a)
Answer to Problem 25.47EP
NAD+ is involved in (3) both glycerol metabolism and fatty acid metabolism.
Explanation of Solution
The first stage of glycerol metabolism is a two-step process. In step 1, glycerol-3-phosphate is formed as the intermediate compound that further reacts to form dihydroxyacetone phosphate in step 2. The reaction for the conversion of glycerol is as follows:
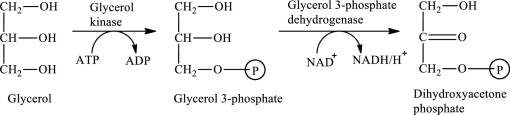
Here, represents
represents
In step 2 of glycerol metabolism, NAD+ oxidized glycerol-3-phosphate to dihydroxyacetone phosphate. Therefore, NAD+ is involved in glycerol metabolism.
The reaction in step 3 of a turn of the β-oxidation pathway is a dehydrogenation reaction in which two hydrogen atoms are removed from L-β-hydroxyacyl CoA. In this reaction, the β-hydroxy group is converted to a β-keto group. NAD+ is used as an oxidizing agent. This reaction is catalyzed by β-hydroxyacyl CoA dehydrogenase enzyme. The reaction for step 3 is as follows:
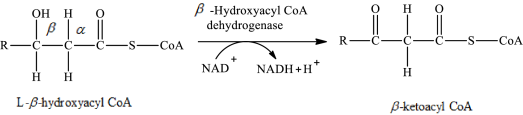
Therefore, NAD+ is involved in (3) both glycerol metabolism and fatty acid metabolism.
(b)
Interpretation:
Whether ADP is involved in (1) glycerol metabolism to dihydroxyacetone phosphate, (2) fatty acid metabolism to acetyl CoA, or (3) both glycerol metabolism and fatty acid metabolism has to be determined.
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of carboxylic acid. They are building blocks of fat in humans and animals.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
Adenosine diphosphate (ADP) provides energy to carry out the metabolic processes in the living cells.
(b)
Answer to Problem 25.47EP
ADP is involved in (1) glycerol metabolism to dihydroxyacetone phosphate.
Explanation of Solution
The first stage of glycerol metabolism is a two-step process. In step 1, glycerol-3-phosphate is formed as the intermediate compound that further reacts to form dihydroxyacetone phosphate in step 2. The reaction for the conversion of glycerol is as follows:
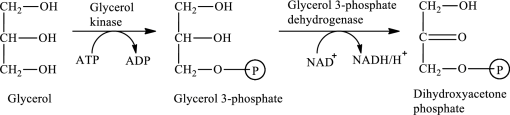
Here, represents
represents
In step 1 of glycerol metabolism, ATP is converted to ADP. Therefore, ADP is involved in glycerol metabolism.
(c)
Interpretation:
Whether kinase is involved in (1) glycerol metabolism to dihydroxyacetone phosphate, (2) fatty acid metabolism to acetyl CoA, or (3) both glycerol metabolism and fatty acid metabolism has to be determined.
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of carboxylic acid. They are building blocks of fat in humans and animals.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
The transfer of a phosphoryl group
(c)
Answer to Problem 25.47EP
Kinase is involved in (1) glycerol metabolism to dihydroxyacetone phosphate.
Explanation of Solution
The first stage of glycerol metabolism is a two-step process. In step 1, glycerol-3-phosphate is formed as the intermediate compound that further reacts to form dihydroxyacetone phosphate in step 2. The reaction for the conversion of glycerol is as follows:
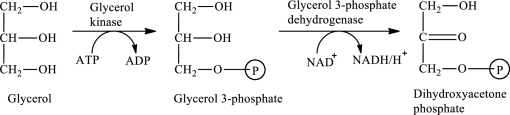
Here, represents
represents
In step 1 of glycerol metabolism, glycerol kinase enzyme catalyzed the conversion of glycerol to glycerol-3-phosphate. Therefore, the kinase is involved in glycerol metabolism.
(d)
Interpretation:
Whether ketoacyl CoA is involved in (1) glycerol metabolism to dihydroxyacetone phosphate, (2) fatty acid metabolism to acetyl CoA, or (3) both glycerol metabolism and fatty acid metabolism has to be determined.
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of carboxylic acid. They are building blocks of fat in humans and animals.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
Nicotinamide adenine dinucleotide is associated with the redox reactions in metabolism. Its reduced form is NADH and oxidized form is NAD+.
(d)
Answer to Problem 25.47EP
Ketoacyl CoA is involved in (2) fatty acid metabolism to acetyl CoA.
Explanation of Solution
The reaction in step 3 of a turn of the β-oxidation pathway is a dehydrogenation reaction in which two hydrogen atoms are removed from L-β-hydroxyacyl CoA. In this reaction, the β-hydroxy group is converted to a β-keto group. NAD+ is used as an oxidizing agent. This reaction is catalyzed by β-hydroxyacyl CoA dehydrogenase enzyme. The reaction for step 3 is as follows:
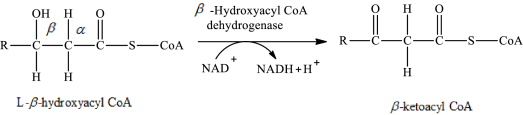
Therefore, ketoacyl CoA is involved in (2) fatty acid metabolism to acetyl CoA.
Want to see more full solutions like this?
Chapter 25 Solutions
General, Organic, and Biological Chemistry
- Propose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forward
- b. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forward
- Identify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



