
Concept explainers
(a)
Interpretation:
The process that has four distinct reaction steps among (1) the β-oxidation pathway, (2) ketogenesis, or (3) lipogenesis has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway.
Ketogenesis is a
(a)
Answer to Problem 25.99EP
The β-oxidation pathway, ketogenesis and chain process of lipogenesis involve four distinct reaction steps.
Explanation of Solution
All three processes involve four distinct reactions.
The four distinct reactions involved in β-oxidation pathway is:
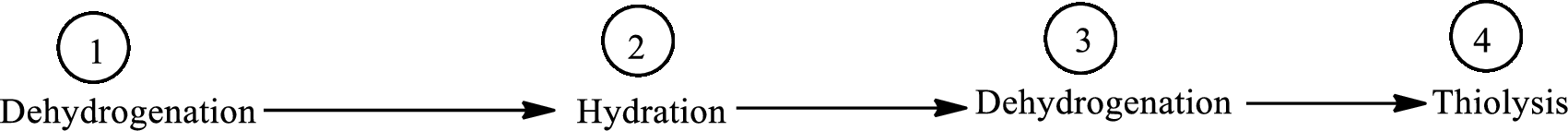
The four distinct reactions involved in lipogenesis is:

The four distinct reactions involved in ketogenesis is:

(b)
Interpretation:
The process that two different hydrogenation reactions among (1) the β-oxidation pathway, (2) ketogenesis, or (3) lipogenesis has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway.
Ketogenesis is a metabolic process by which ketone bodies are produced by the breakdown of fatty acids and ketogenic amino acids. This metabolic process supplies our organs with needed energy under certain circumstances such as starvation. Fatty acid molecules degrade into acetyl CoA which are utilized as reactants in the process of ketogenesis. These molecules of acetyl CoA undergo the process of condensation twice, followed by chain cleavage and hydrogenation to produce ketone bodies.
In hydrogenation reaction, a hydrogen molecule (H2) is added to an organic substance.
(b)
Answer to Problem 25.99EP
Lipogenesis has two different hydrogenation reactions.
Explanation of Solution
Lipogenesis involves two different hydrogenation reactions in step 2 and 4 of the cyclic process of lipogenesis.
Step 2 is the first hydrogenation reaction step of the cyclic process and it involves the hydrogenation of acetoacetyl ACP to synthesis β-hydroxybutyryl ACP with the help of reducing agent NADPH. The reaction of this step is:
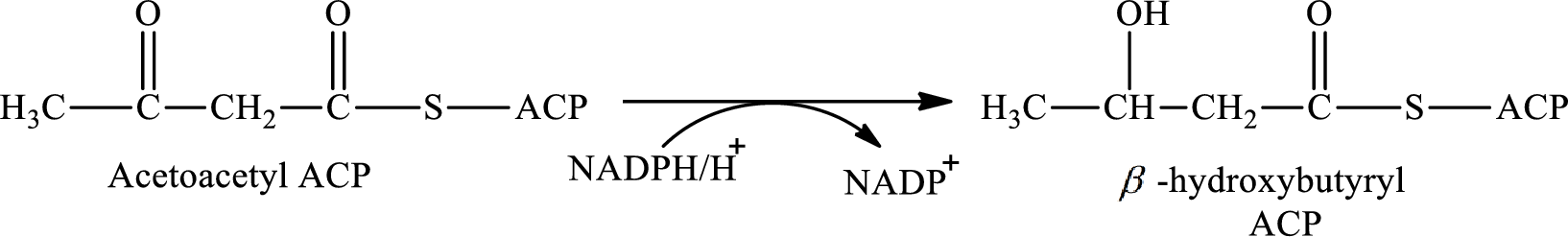
Step 4 involves the hydrogenation reaction. In this step, crotonyl ACP is converted to butyryl ACP with the help of reducing agent NADPH. The reaction of this step is:
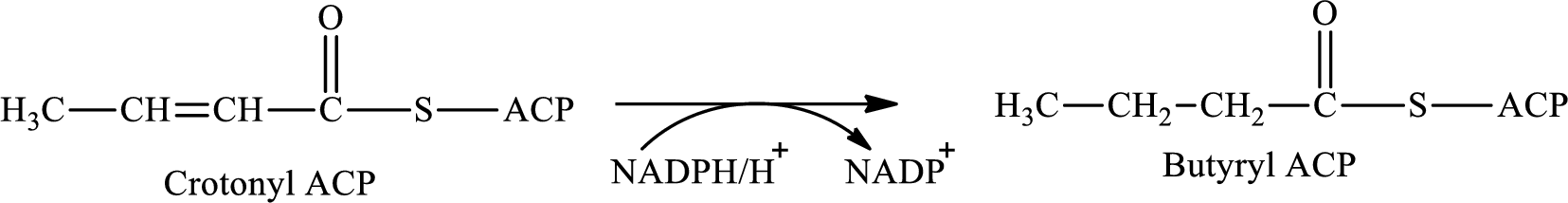
(c)
Interpretation:
The process that two different dehydrogenation reactions among (1) the β-oxidation pathway, (2) ketogenesis, or (3) lipogenesis has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway.
Ketogenesis is a metabolic process by which ketone bodies are produced by the breakdown of fatty acids and ketogenic amino acids. This metabolic process supplies our organs with needed energy under certain circumstances such as starvation. Fatty acid molecules degrade into acetyl CoA which are utilized as reactants in the process of ketogenesis. These molecules of acetyl CoA undergo the process of condensation twice, followed by chain cleavage and hydrogenation to produce ketone bodies.
In dehydrogenation reaction, a hydrogen molecule (H2) is removed from an organic substance.
(c)
Answer to Problem 25.99EP
The β-oxidation pathway has two different dehydrogenation reactions.
Explanation of Solution
The β-oxidation pathway has two different dehydrogenation reactions in step 1 and 3.
In step 1 of a turn of the β-oxidation pathway, hydrogen atoms from
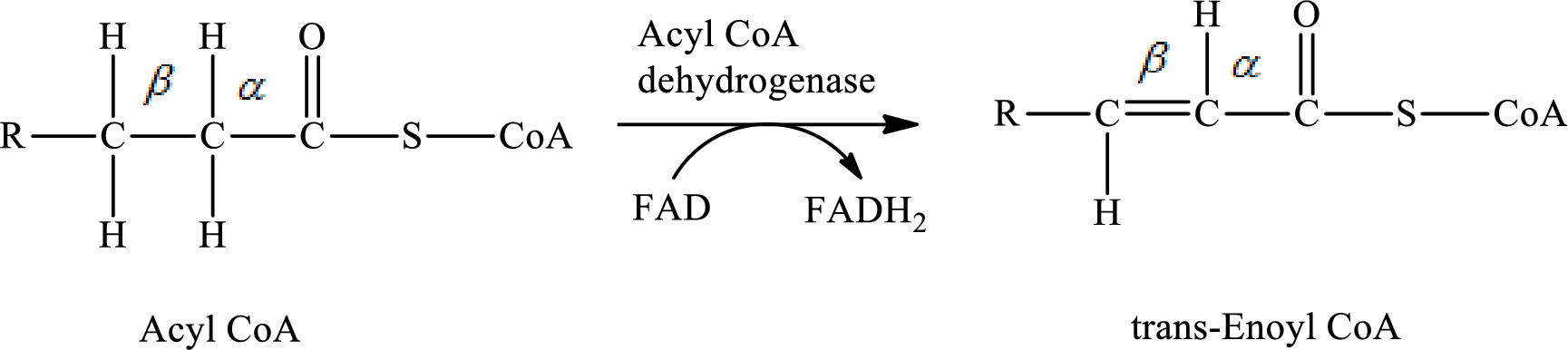
In step 3 of a turn of the β-oxidation pathway, two hydrogen atoms are removed from L- β-hydroxyacyl CoA. In this reaction, β-hydroxy group is converted to a keto group. NAD+ is used as an oxidizing agent. This reaction is catalyzed by β-hydroxyacyl CoA dehydrogenase enzyme. The reaction for step 3 is as follows:
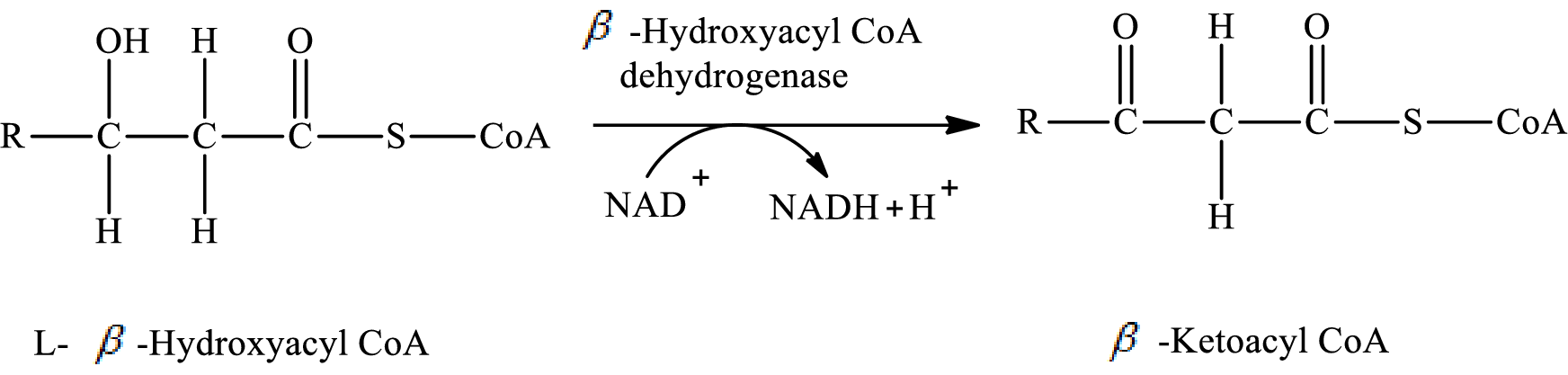
(d)
Interpretation:
The process that involves thiolysis reaction among (1) the β-oxidation pathway, (2) ketogenesis, or (3) lipogenesis has to be identified.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway.
Ketogenesis is a metabolic process by which ketone bodies are produced by the breakdown of fatty acids and ketogenic amino acids. This metabolic process supplies our organs with needed energy under certain circumstances such as starvation. Fatty acid molecules degrade into acetyl CoA which are utilized as reactants in the process of ketogenesis. These molecules of acetyl CoA undergo the process of condensation twice, followed by chain cleavage and hydrogenation to produce ketone bodies.
In thiolysis reaction, thiol (-SH) group cleaves one compound into two compounds.
(d)
Answer to Problem 25.99EP
The β-oxidation pathway involves thiolysis reaction.
Explanation of Solution
In step 4 of a turn of the β-oxidation pathway, the fatty acid carbon chain between
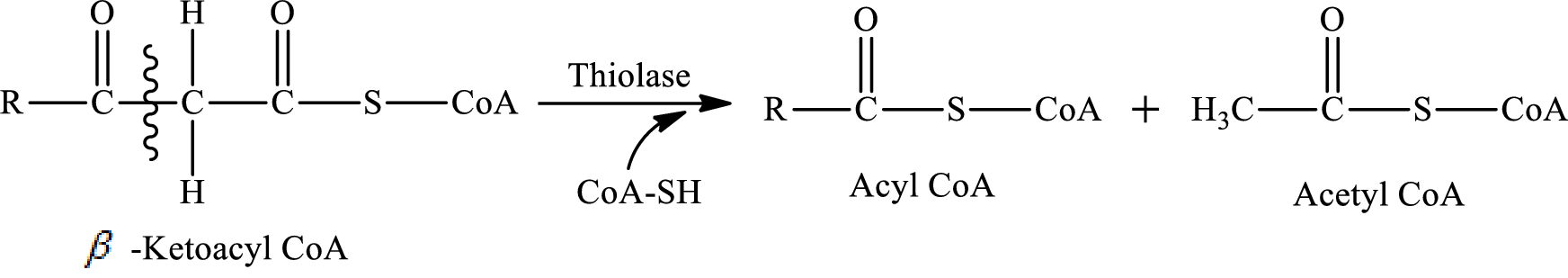
The carbon-carbon bond in β-ketoacyl CoA is cleaved by CoA-SH molecule. Acyl CoA and acetyl CoA is formed in this reaction.
Want to see more full solutions like this?
Chapter 25 Solutions
General, Organic, and Biological Chemistry
- i need help with the followingarrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO(g) +Cl₂ (g) = 2NOC1 (g) AGº = -41. kJ Now suppose a reaction vessel is filled with 8.90 atm of chlorine (C12) and 5.71 atm of nitrosyl chloride (NOC1) at 1075. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. atm ☑ 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCN is a weak acid. acids: 0.29 mol of NaOH is added to 1.0 L of a 1.2M HCN solution. bases: ☑ other: 0.09 mol of HCl is added to acids: 1.0 L of a solution that is bases: 0.3M in both HCN and KCN. other: 0,0,... ? 00. 18 Ar 日arrow_forward
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: 0.2 mol of KOH is added to 1.0 L of a 0.5 M HF solution. bases: Х other: ☐ acids: 0.10 mol of HI is added to 1.0 L of a solution that is 1.4M in both HF and NaF. bases: other: ☐ 0,0,... ด ? 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. acids: ☐ 1.8 mol of HCl is added to 1.0 L of a 1.0M NH3 bases: ☐ solution. other: ☐ 0.18 mol of HNO3 is added to 1.0 L of a solution that is 1.4M in both NH3 and NH₁Br. acids: bases: ☐ other: ☐ 0,0,... ? 000 18 Ar B 1arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NH3 (g) = N2 (g) +3H₂ —N2 (g) AGº = 34. kJ Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH 3 tend to rise or fall? ☐ x10 fall Х Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH 3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no atm 00. 18 Ar 무ㅎ ?arrow_forward
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forwardHomework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forward
- Identifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning




