
Indicate whether each of the following aspects of triacylglycerol digestion is associated with the (1) mouth, (2) stomach, (3) small intestine, (4) intestinal cells, or (5) bloodstream.
- a. Chyme is produced.
- b. Gastric lipases are active.
- c. Initial production of monoacylglycerols occurs.
- d. Fatty acid micelles are formed.
(a)
Interpretation: To identify whether the chyme is produced in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: In the stomach, the muscle walls of the stomach churn the food and other complex molecules into smaller particles. The churning action breaks up the lipid molecules into small droplets/globules which remains suspended on the other smaller components of the ingested food. This results in the formation of a semi-liquid material comprising of small lipid droplets, partly digested food and gastric secretions called chyme. The chyme, thereafter, resumes its journey to the small intestine.
Answer to Problem 25.1EP
Chyme production occurs in the stomach.
Explanation of Solution
Chyme is a semi-liquid material composed of small lipid droplets, partly digested food and gastric secretions. Since the initial breakdown of lipid molecules and activity of the gastric lipases begins in the stomach, hence the formation of chyme occurs in the stomach.
(b)
Interpretation: To identify whether the gastric lipase is active in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: Gastric lipase is an acidic lipase secreted by the gastric chief cells in the fundic mucosa in the stomach. The function of the enzyme is to break down lipid molecules. Gastric lipases are enzymes which break down lipids.
Answer to Problem 25.1EP
Gastric lipases are active in the stomach.
Explanation of Solution
Gastric lipases are present in the stomach and help in the initial hydrolysis of the triacylglycerol molecules. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). Around 10% of the triacylglycerols are hydrolyzed in the stomach by the action of gastric lipases. Hence, gastric lipases are active in the stomach.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
(c)
Interpretation: To identify whether the initial production of monoacylglycerol occurs in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: The initial lipid digestion begins in the stomach in the presence of gastric lipases. Gastric lipases are enzymes which break down lipids. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. Here the digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
Answer to Problem 25.1EP
The initial production of monoacylglycerol occurs in the stomach.
Explanation of Solution
In the stomach, around 10% of the triacylglycerol is hydrolyzed into monoacylglycerol and free fatty acids under the activity of gastric lipases. The remaining hydrolysis occurs in the small intestine. Hence, the initial production of monoacylglycerol occurs in the stomach.
(d)
Interpretation: To identify whether the fatty acid micelles are formed in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: Fatty acid is a carboxylic acid that contains a long hydrophobic hydrocarbon chain and a polar carboxyl group. The fatty acids are aligned such that the hydrophobic hydrocarbon chain is directed inwards away from the polar environment and the polar carboxyl group head is directed outwards. The aggregate thus formed is called micelle.
A general representation of fatty acid is,
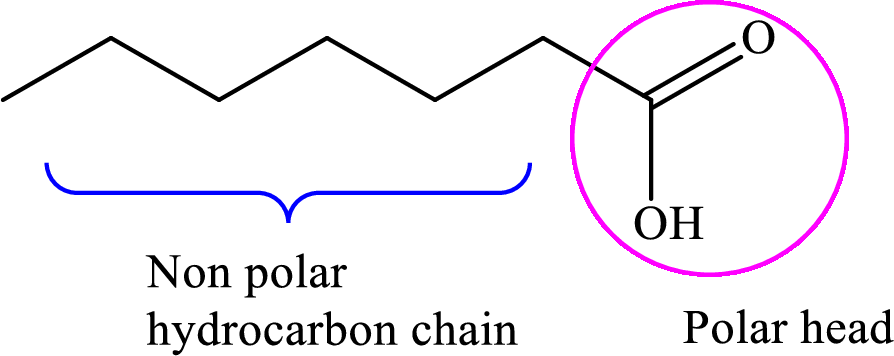
A diagrammatic representation of micelle is as follows:
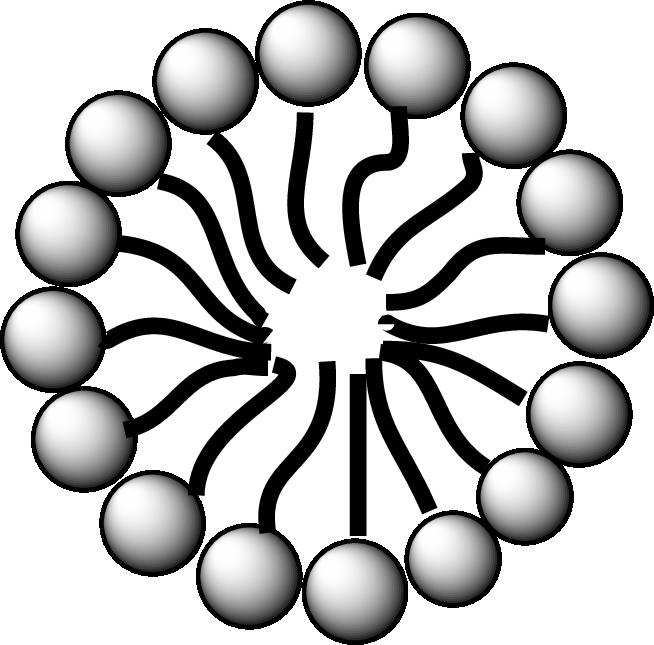
Answer to Problem 25.1EP
Fatty acid micelles are formed in the small intestine.
Explanation of Solution
A fatty acid micelle is a spherical droplet which is primarily composed of free fatty acids, monoacylglycerols, and bile.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
In the small intestine, the monoacylglycerols and free fatty acids along with bile and pancreatic lipases, form micelles. Micelles are later absorbed by the cell membranes of the intestinal cells.
Want to see more full solutions like this?
Chapter 25 Solutions
General, Organic, and Biological Chemistry
- Complete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forwardThe statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forwardComplete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forward
- Show the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forward
- Draw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forwardQ5: Propose a reasonable synthesis for the following decalin derivative. using only decalin and alkanes of 3 or fewer carbons. Decalin H3C HO க CH3arrow_forward
- 2Helparrow_forwardplease add appropriate arrows, and tell me clearly where to add arrows, or draw itarrow_forwardWhat I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





