
Concept explainers
(a)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate butyrate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of
(a)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid butyrate intermediate is succinate.
Explanation of Solution
Intermediates involved in the lipogenesis are derivative of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
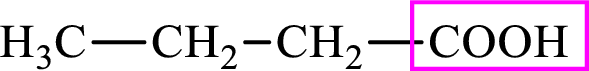
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a
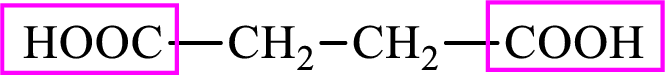
The structure of butyrate is,
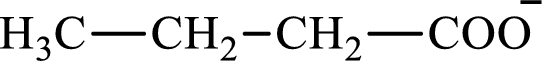
The structure of succinate is,
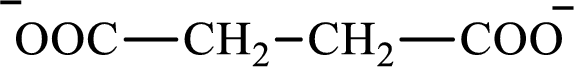
Butyrate and succinate are saturated acids with four carbon atoms in each molecule. Butyrate is a monoacid that is formed as an intermediate in lipogenesis while succinate is a diacid that is formed as an intermediate in the citric acid cycle. Therefore, the citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid butyrate intermediate is succinate.
(b)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate acetoacetate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of redox reactions.
Intermediates involved in the lipogenesis are derivatives of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
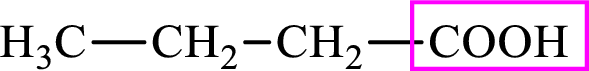
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a C4 derivative of dicarboxylic acid. The structure of succinic acid is,
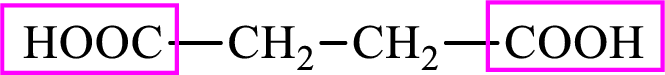
(b)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid acetoacetate intermediate is oxaloacetate.
Explanation of Solution
Acetoacetate is the intermediate in the lipogenesis. The structure of acetoacetate is,
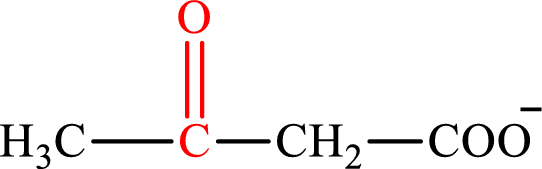
Oxaloacetate is the intermediate in the citric acid cycle. The structure of oxaloacetate is,
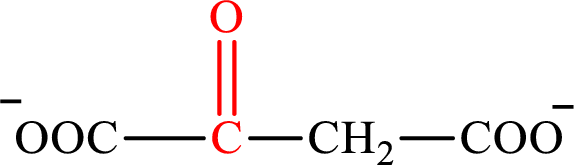
Acetoacetate and oxaloacetate are the keto derivatives of saturated acid with four carbon atoms in each molecule. Acetoacetate is a keto derivative of monocarboxylic acid while oxaloacetate is a keto derivative of dicarboxylic acid. Therefore, the citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid acetoacetate intermediate is oxaloacetate.
(c)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate β-hydroxybutyrate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of redox reactions.
Intermediates involved in the lipogenesis are derivatives of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
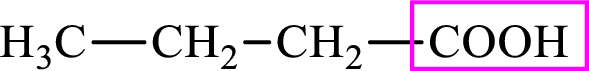
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a C4 derivative of dicarboxylic acid. The structure of succinic acid is,
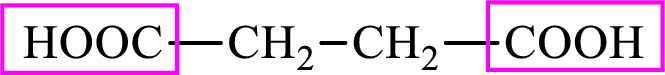
(c)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid β-hydroxybutyrate intermediate is malate.
Explanation of Solution
β-Hydroxybutyrate is the intermediate in the lipogenesis. The structure of β-hydroxybutyrate is,
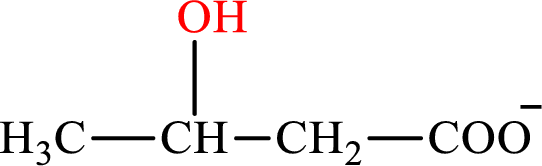
Malate is the intermediate in the citric acid cycle. The structure of Malate is,
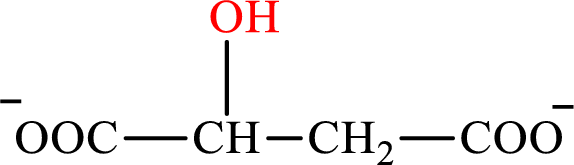
β-Hydroxybutyrate and malate are the hydroxy derivatives of saturated acid with four carbon atoms in each molecule. β-Hydroxybutyrate is a hydroxy derivative of monocarboxylic acid while malate is a hydroxy derivative of dicarboxylic acid. Therefore, the citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid β-hydroxybutyrate intermediate is malate.
(d)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate crotonate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of redox reactions.
Intermediates involved in the lipogenesis are derivatives of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
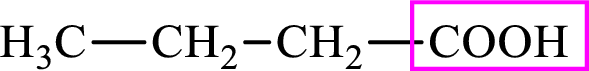
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a C4 derivative of dicarboxylic acid. The structure of succinic acid is,
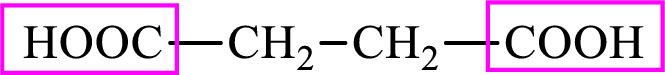
(d)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid crotonate intermediate is fumarate.
Explanation of Solution
Crotonate is the intermediate in the lipogenesis. The structure of crotonate is,
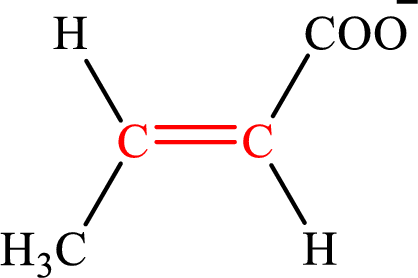
Fumarate is the intermediate in the citric acid cycle. The structure of fumarate is,
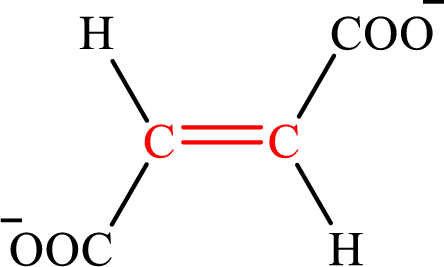
Crotonate and fumarate are the unsaturated derivatives of a
Want to see more full solutions like this?
Chapter 25 Solutions
General, Organic, and Biological Chemistry
- You have identified a new species of a Gram-positive bacteria. You would like to screen their genome for all proteins that are covalently linked to the cell wall. You have annotated the genome, so that you identified all the promoters, operons, and genes sequences within the operons. Using these features, what would you screen for to identify a set of candidates for proteins covalently linked to the bacterial cell wall.arrow_forwardBelow is a diagram from a genomic locus of a bacterial genome. Each arrow represents a coding region, and the arrowheads indicate its orientation in the genome. The numbers are randomly assigned. Draw the following features on the diagram, and explain your rationale for each feature: 10 12 合會會會會長 6 a) Expected transcriptions, based on known properties of bacterial genes and operons. How many proteins are encoded in each of the transcripts? b) Location of promoters (include rationale) c) Location of transcriptional terminators (include rationale) d) Locations of Shine-Dalgarno sequences (include rationale)arrow_forwardSample excuse letter in school class for the reasons of headaches and dysmenorrhea caused by menstrual cyclearrow_forward
- How do the muscles on the foot work to balance on an ice skate, specifically the triangle of balance and how does it change when balancing on an ice skate? (Refer to anatomy, be specific)arrow_forwardWhich of the following is NOT an example of passive immunization? A. Administration of tetanus toxoid B. Administration of hepatitis B immunoglobulin C. Administration of rabies immunoglobulin D. Transfer of antibodies via plasma therapyarrow_forwardTranscription and Translation 1. What is the main function of transcription and translation? (2 marks) 2. How is transcription different in eukaryotic and prokaryotic cells? (2 marks) 3. Explain the difference between pre-mRNA and post-transcript mRNA. (2 marks) 4. What is the function of the following: (4 marks) i. the cap ii. spliceosome iii. Poly A tail iv. termination sequence 5. What are advantages to the wobble feature of the genetic code? (2 marks) 6. Explain the difference between the: (3 marks) i. A site & P site ii. codon & anticodon iii. gene expression and gene regulation 7. Explain how the stop codon allows for termination. (1 mark) 8. In your own words, summarize the process of translation. (2 marks)arrow_forward
- In this activity you will research performance enhancers that affect the endocrine system or nervous system. You will submit a 1 page paper on one performance enhancer of your choice. Be sure to include: the specific reason for use the alleged results on improving performance how it works how it affect homeostasis and improves performance any side-effects of this substancearrow_forwardNeurons and Reflexes 1. Describe the function of the: a) dendrite b) axon c) cell body d) myelin sheath e) nodes of Ranvier f) Schwann cells g) motor neuron, interneuron and sensory neuron 2. List some simple reflexes. Explain why babies are born with simple reflexes. What are they and why are they necessary. 3. Explain why you only feel pain after a few seconds when you touch something very hot but you have already pulled your hand away. 4. What part of the brain receives sensory information? What part of the brain directs you to move your hand away? 5. In your own words describe how the axon fires.arrow_forwardMutations Here is your template DNA strand: CTT TTA TAG TAG ATA CCA CAA AGG 1. Write out the complementary mRNA that matches the DNA above. 2. Write the anticodons and the amino acid sequence. 3. Change the nucleotide in position #15 to C. 4. What type of mutation is this? 5. Repeat steps 1 & 2. 6. How has this change affected the amino acid sequence? 7. Now remove nucleotides 13 through 15. 8. Repeat steps 1 & 2. 9. What type of mutation is this? 0. Do all mutations result in a change in the amino acid sequence? 1. Are all mutations considered bad? 2. The above sequence codes for a genetic disorder called cystic fibrosis (CF). 3. When A is changed to G in position #15, the person does not have CF. When T is changed to C in position #14, the person has the disorder. How could this have originated?arrow_forward
- hoose a scientist(s) and research their contribution to our derstanding of DNA structure or replication. Write a one page port and include: their research where they studied and the time period in which they worked their experiments and results the contribution to our understanding of DNA cientists Watson & Crickarrow_forwardhoose a scientist(s) and research their contribution to our derstanding of DNA structure or replication. Write a one page port and include: their research where they studied and the time period in which they worked their experiments and results the contribution to our understanding of DNA cientists Watson & Crickarrow_forward7. Aerobic respiration of a protein that breaks down into 12 molecules of malic acid. Assume there is no other carbon source and no acetyl-CoA. NADH FADH2 OP ATP SLP ATP Total ATP Show your work using dimensional analysis here: 3arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax





