
Concept explainers
How does the structure of a cycloalkane differ from that of a straight-chain or branched-chain
Interpretation:
The difference in structure of cycloalkane from that of a straight chain or branched-chain alkane needs to be explained.
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkanes.
Answer to Problem 53A
The difference in structure of cycloalkane from that of a straight chain or branched-chain alkane is that cycloalkanes contains 2 H atoms less than that of straight chain or branched-chain alkane.
Explanation of Solution
The general formula of alkane is
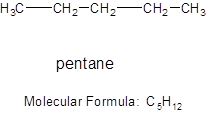
The branched chain of 5 carbon atoms will be:
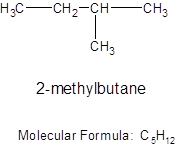
For a cycloalkane formed from 5 carbon atoms, the structure and the molecular formula will be:
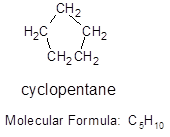
Cyclopentane that means it contains 5 carbon atoms and 10 hydrogen atoms.
Since, in cycloalkane the number of hydrogen atoms are less from alkane by 2 units so, the general formula of cycloalkane will be:
Hence, the difference in structure of cycloalkane from that of a straight chain or branched-chain alkane is that cycloalkanes contains 2 H atoms less than that of straight chain or branched-chain alkane.
Chapter 21 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: The Central Science (14th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Inorganic Chemistry
Organic Chemistry
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





