
Concept explainers
Draw all the structural isomers for C8H18 that have the following root name (longest carbon chain). Name the structural isomers.
a. hexane
b. pentane
(a)
Interpretation: The structural isomers of
Concept introduction: Rules given by IUPAC should be followed to name an organic compound. Any organic compound has only one name that denotes that compound. The root word determines the number of carbons while counting the longest carbon chain. If more than one substituent is present, prefixes like di, tri, tetra, etc. are used and different substituents are written in alphabetical order.
Answer to Problem 16E
Answer
The structural isomers of
Explanation of Solution
Explanation
To determine: The structural isomers of
The structural isomer is given below and its name is
The structure of the isomer is,
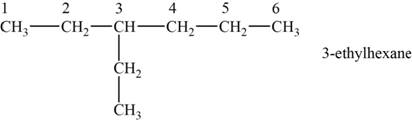
Figure 1
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Ethyl group is attached to third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
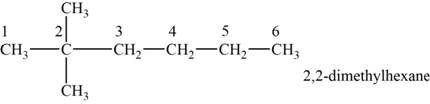
Figure 2
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Two methyl groups are attached to second carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the given isomer is,
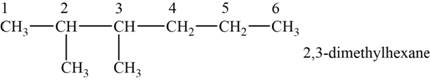
Figure 3
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
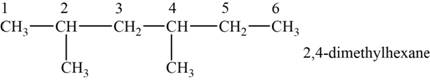
Figure 4
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and fourth carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
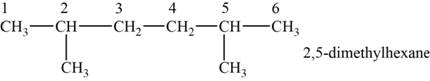
Figure 5
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and fifth carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
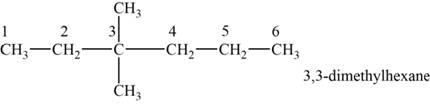
Figure 6
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Two Methyl groups are attached to third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
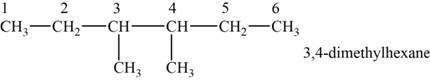
Figure 7
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl groups are attached to third and fourth carbon, thus the name of the isomer is
Conclusion
The structural isomers of
(b)
Interpretation: The structural isomers of
Concept introduction: Rules given by IUPAC should be followed to name an organic compound. Any organic compound has only one name that denotes that compound. The root word determines the number of carbons while counting the longest carbon chain. If more than one substituent is present, prefixes like di, tri, tetra, etc. are used and different substituents are written in alphabetical order.
Explanation of Solution
Explanation
To determine: The structural isomers of
The structural isomer is given below and its name is
The structure of the isomer is,
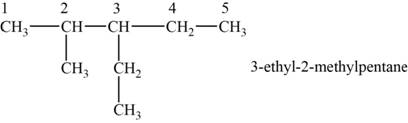
Figure 8
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group is attached to second carbon, ethyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
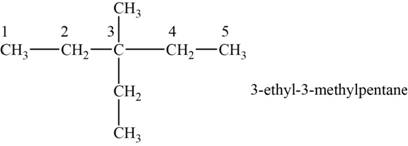
Figure 9
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group and ethyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
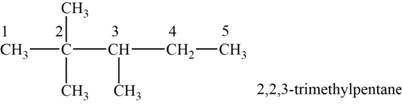
Figure 10
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to second carbon and one methyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
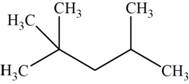
Figure 11
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to second carbon and one methyl group is attached to fourth carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
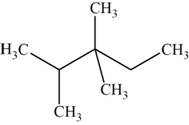
Figure 12
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to third carbon and one methyl group is attached to second carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
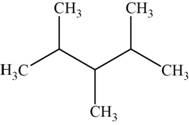
Figure 13
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group is attached to second, third carbon and fourth carbon, therefore, the name of the isomer is
Conclusion
The structural isomers of
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry: An Atoms First Approach
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





