
Chemistry: An Atoms First Approach
2nd Edition
ISBN: 9781305079243
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 50E
Minoxidil (C9H15N5O) is a compound produced by Pharmacia Company that has been approved as a treatment of some types of male pattern baldness.
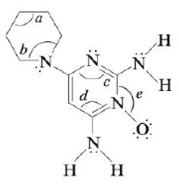
a. Would minoxidil be more soluble in acidic or basic aqueous solution? Explain.
b. Give the hybridization of the five nitrogen atoms in minoxidil.
c. Give the hybridization of each of the nine carbon atoms in minoxidil.
d. Give approximate values of the bond angles marked a, b, c, d, and e.
e. Including all the hydrogen atoms, how many σ bonds exist in minoxidil?
f. How many π bonds exist in minoxidil?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How to name hydrocarbons
Please do these questions within the SCH4U course please with full steps since I am still unsure how to format my answers! Thank you so much.
When two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)
Chapter 21 Solutions
Chemistry: An Atoms First Approach
Ch. 21 - What is a hydrocarbon? What is the difference...Ch. 21 - Prob. 2RQCh. 21 - Prob. 3RQCh. 21 - Summarize the nomenclature rules for alkanes,...Ch. 21 - What functional group distinguishes each of the...Ch. 21 - Distinguish between isomerism and resonance....Ch. 21 - Prob. 7RQCh. 21 - Prob. 8RQCh. 21 - Prob. 9RQCh. 21 - Prob. 10RQ
Ch. 21 - Prob. 11RQCh. 21 - Prob. 12RQCh. 21 - Prob. 1QCh. 21 - Prob. 2QCh. 21 - What is wrong with the following names? Give the...Ch. 21 - Prob. 4QCh. 21 - Prob. 5QCh. 21 - Prob. 6QCh. 21 - Prob. 7QCh. 21 - Prob. 8QCh. 21 - Prob. 9QCh. 21 - Prob. 10QCh. 21 - Prob. 11QCh. 21 - Prob. 12QCh. 21 - Prob. 13ECh. 21 - Prob. 14ECh. 21 - Draw all the structural isomers for C8H18 that...Ch. 21 - Draw all the structural isomers for C8H18 that...Ch. 21 - Prob. 17ECh. 21 - Prob. 18ECh. 21 - Draw the structural formula for each of the...Ch. 21 - Prob. 20ECh. 21 - Prob. 21ECh. 21 - Prob. 22ECh. 21 - Prob. 23ECh. 21 - Prob. 24ECh. 21 - Name each of the following alkenes. a. CH2 = CH ...Ch. 21 - Name each of the following alkenes or alkynes. a....Ch. 21 - Prob. 27ECh. 21 - Prob. 28ECh. 21 - Prob. 29ECh. 21 - Prob. 30ECh. 21 - Name each of the following. a. b. CH3CH2CH2CCl3 c....Ch. 21 - Prob. 32ECh. 21 - There is only one compound that is named...Ch. 21 - Prob. 34ECh. 21 - Prob. 35ECh. 21 - Prob. 36ECh. 21 - Prob. 37ECh. 21 - Prob. 38ECh. 21 - Prob. 39ECh. 21 - Prob. 40ECh. 21 - Draw all structural and geometrical (cistrans)...Ch. 21 - Prob. 42ECh. 21 - Prob. 43ECh. 21 - Prob. 44ECh. 21 - If one hydrogen in a hydrocarbon is replaced by a...Ch. 21 - There are three isomers of dichlorobenzene, one of...Ch. 21 - Prob. 47ECh. 21 - Prob. 48ECh. 21 - Prob. 49ECh. 21 - Minoxidil (C9H15N5O) is a compound produced by...Ch. 21 - Prob. 51ECh. 21 - Prob. 52ECh. 21 - Name all the alcohols that have the formula...Ch. 21 - Prob. 54ECh. 21 - Prob. 55ECh. 21 - Prob. 56ECh. 21 - Prob. 57ECh. 21 - Prob. 58ECh. 21 - Prob. 59ECh. 21 - Prob. 60ECh. 21 - Prob. 61ECh. 21 - Prob. 62ECh. 21 - Prob. 63ECh. 21 - Prob. 64ECh. 21 - Prob. 65ECh. 21 - Prob. 66ECh. 21 - Prob. 67ECh. 21 - Prob. 68ECh. 21 - Prob. 69ECh. 21 - Complete the following reactions. a. CH3CO2H +...Ch. 21 - Prob. 71ECh. 21 - Prob. 72ECh. 21 - Prob. 73ECh. 21 - Prob. 74ECh. 21 - Prob. 75ECh. 21 - The polyester formed from lactic acid, is used for...Ch. 21 - Prob. 77ECh. 21 - Prob. 78ECh. 21 - Prob. 79ECh. 21 - Prob. 80ECh. 21 - Prob. 81ECh. 21 - Prob. 82ECh. 21 - Prob. 83ECh. 21 - Prob. 84ECh. 21 - Prob. 85ECh. 21 - Prob. 86ECh. 21 - Prob. 87ECh. 21 - Prob. 88ECh. 21 - Prob. 89ECh. 21 - Prob. 90ECh. 21 - Prob. 91ECh. 21 - Prob. 92ECh. 21 - Prob. 93ECh. 21 - Prob. 94ECh. 21 - Prob. 95ECh. 21 - Prob. 96ECh. 21 - Prob. 97ECh. 21 - Prob. 98ECh. 21 - Prob. 99ECh. 21 - Prob. 100ECh. 21 - Prob. 101ECh. 21 - Prob. 102ECh. 21 - Prob. 103ECh. 21 - Prob. 104ECh. 21 - Prob. 105ECh. 21 - Prob. 106ECh. 21 - Which base will hydrogen-bond with uracil within...Ch. 21 - Prob. 108ECh. 21 - The base sequences in mRNA that code for certain...Ch. 21 - Prob. 110ECh. 21 - Prob. 111AECh. 21 - Prob. 112AECh. 21 - Prob. 113AECh. 21 - Prob. 114AECh. 21 - Prob. 115AECh. 21 - Prob. 116AECh. 21 - Prob. 117AECh. 21 - Prob. 118AECh. 21 - Prob. 119AECh. 21 - Prob. 120AECh. 21 - Prob. 121AECh. 21 - Prob. 122AECh. 21 - Prob. 123AECh. 21 - Prob. 124AECh. 21 - Prob. 125AECh. 21 - Prob. 126AECh. 21 - Prob. 127AECh. 21 - Prob. 128AECh. 21 - Prob. 129AECh. 21 - Prob. 130AECh. 21 - Prob. 131AECh. 21 - Prob. 132AECh. 21 - Prob. 133AECh. 21 - Prob. 134AECh. 21 - When heat is added to proteins, the hydrogen...Ch. 21 - Prob. 136AECh. 21 - Prob. 137CWPCh. 21 - Prob. 138CWPCh. 21 - Prob. 139CWPCh. 21 - Name each of the following alkenes and alkynes. a....Ch. 21 - a. Name each of the following alcohols. b. Name...Ch. 21 - Prob. 142CWPCh. 21 - Prob. 143CWPCh. 21 - Prob. 144CWPCh. 21 - Prob. 145CPCh. 21 - Prob. 146CPCh. 21 - Prob. 147CPCh. 21 - Prob. 148CPCh. 21 - Prob. 149CPCh. 21 - Prob. 150CPCh. 21 - Prob. 151CPCh. 21 - Prob. 152CPCh. 21 - Prob. 153CPCh. 21 - Prob. 154CPCh. 21 - Stretch a rubber band while holding it gently to...Ch. 21 - Alcohols are very useful starting materials for...Ch. 21 - Prob. 157CPCh. 21 - Prob. 158CPCh. 21 - Prob. 159IPCh. 21 - Prob. 160IPCh. 21 - Prob. 161MPCh. 21 - Prob. 162MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forwardGiven the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forwardThe decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward
- 2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forwardThe emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forwardIndicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forward
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY