
Concept explainers
(a)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective
To determine: The correct name of the given compound and its structure is to be drawn.
(a)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of
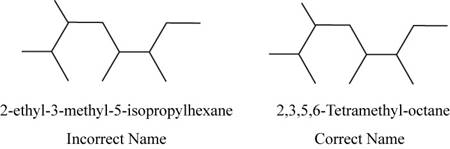
Figure 1
The root name hexane signifies the presence of six carbon atoms.
(b)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective functional groups. When they are named the root term, suffix and prefix are to be remembered. They are named according to the International Union of Pure and Applied Chemistry
To determine: The correct name of the given compound and its structure is to be drawn.
(b)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of
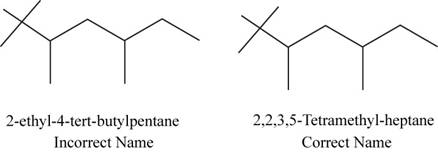
Figure 2
The root name is pentane which signifies the presence of five carbon atoms in a chain,
(c)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective functional groups. When they are named the root term, suffix and prefix are to be remembered. They are named according to the International Union of Pure and Applied Chemistry
To determine: The correct name of the given compound and its structure is to be drawn.
(c)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of 3-methyl 4-isopropylpentane with its correct name is,
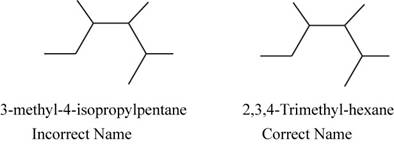
Figure 3
The root name pentane signifies the presence of five carbon atoms in a chain and
(d)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective functional groups. When they are named the root term, suffix and prefix are to be remembered. They are named according to the International Union of Pure and Applied Chemistry
To determine: The correct name of the given compound and its structure is to be drawn.
(d)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of
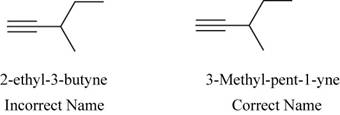
Figure 4
The root name butyne signifies the presence of four carbon atoms with a triple bond in a chain and
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry: An Atoms First Approach
- For the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forwardCan I please get this answered? With the correct number of significant digits.arrow_forwardDraw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





